The Role of Dileucine in the Expression and Function of Human Organic Anion Transporter 1 (hOAT1)
- PMID: 21494320
- PMCID: PMC3008821
The Role of Dileucine in the Expression and Function of Human Organic Anion Transporter 1 (hOAT1)
Abstract
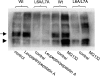
Human organic anion transporter hOAT1 plays a critical role in the body disposition of environmental toxins and clinically important drugs including anti-HIV therapeutics, anti-tumor drugs, antibiotics, anti-hypertensives, and anti-inflammatories. In the current study, we investigated the role of dileucine (L6L7) at the amino terminus of hOAT1 in the expression and function of the transporter. We substituted L6L7 with alanine (A) simultaneously. The resulting mutant transporter L6A/L7A showed no transport activity due to its complete loss of expression at the cell surface. Such loss of surface expression of L6A/L7A was consistent with a complete loss of an 80 kDa mature form and a dramatic decrease in a 60 kDa immature form of the mutant transporter in the total cell lysates. Treatment of L6A/L7A-expressing cells with proteasomal inhibitor resulted in a significant increase in the immature form of hOAT1, but not its mature form, whereas treatment of these cells with lysosomal inhibitor had no effect on the expression of the mutant transporters, suggesting that the mutant transporter was degraded through proteasomal pathway. The accumulation of mutant transporter in the endoplasmic reticulum (ER) was confirmed by coimmunolocalization of L6L7 with calnexin, an ER marker. Furthermore, treatment of L6A/L7A-expressing cells with sodium 4-phenylbutyrate (4PBA) and glycerol, two chemical chaperones, could not promote the exit of the immature form of the mutant transporter from the ER. Our data suggest that L6L7 are critical for the stability and ER export of hOAT1.
Figures
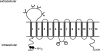



Similar articles
-
Mutational analysis of the role of GXXXG motif in the function of human organic anion transporter 1 (hOAT1).Int J Biochem Mol Biol. 2011 Jan 1;2(1):1-7. Int J Biochem Mol Biol. 2011. PMID: 21340049 Free PMC article.
-
Critical amino acid residues in transmembrane domain 1 of the human organic anion transporter hOAT1.J Biol Chem. 2004 Jul 23;279(30):31478-82. doi: 10.1074/jbc.M404686200. Epub 2004 May 15. J Biol Chem. 2004. PMID: 15145940
-
Putative transmembrane domain 12 of the human organic anion transporter hOAT1 determines transporter stability and maturation efficiency.J Pharmacol Exp Ther. 2010 Feb;332(2):650-8. doi: 10.1124/jpet.109.160515. Epub 2009 Nov 5. J Pharmacol Exp Ther. 2010. PMID: 19892921 Free PMC article.
-
Serum- and glucocorticoid-inducible kinase sgk2 stimulates the transport activity of human organic anion transporters 1 by enhancing the stability of the transporter.Int J Biochem Mol Biol. 2016 Jun 1;7(1):19-26. eCollection 2016. Int J Biochem Mol Biol. 2016. PMID: 27335683 Free PMC article.
-
The putative transmembrane segment 7 of human organic anion transporter hOAT1 dictates transporter substrate binding and stability.J Pharmacol Exp Ther. 2007 Mar;320(3):1209-15. doi: 10.1124/jpet.106.117663. Epub 2006 Dec 13. J Pharmacol Exp Ther. 2007. PMID: 17167169
Cited by
-
Transmembrane helix 1 contributes to substrate translocation and protein stability of bile acid transporter SLC10A2.J Biol Chem. 2011 Aug 5;286(31):27322-32. doi: 10.1074/jbc.M110.217802. Epub 2011 Jun 6. J Biol Chem. 2011. PMID: 21646357 Free PMC article.
References
-
- You G. The role of organic ion transporters in drug disposition: an update. Curr Drug Metab. 2004;5:55–62. - PubMed
-
- A nzai N, Kanai Y, Endou H. Organic anion transporter family: current knowledge. J Pharmacol Sci. 2006;100:411–426. - PubMed
-
- El-Sheikh AA, Masereeuw R, Russel FG. Mechanisms of renal anionic drug transport. Eur.J.Pharmacol. 2008;585:245–255. - PubMed
-
- Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem. 1997;272:18526–18529. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
