Methylphenidate transdermal system: a multisite, open-label study of dermal reactions in pediatric patients diagnosed with ADHD
- PMID: 21494336
- PMCID: PMC3067997
- DOI: 10.4088/PCC.10m00996pur
Methylphenidate transdermal system: a multisite, open-label study of dermal reactions in pediatric patients diagnosed with ADHD
Abstract
Objective: To characterize dermal reactions and examine methylphenidate (MPH) sensitization in subjects receiving methylphenidate transdermal system (MTS).
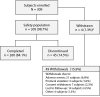
Method: This multicenter, open-label, dose-optimization study utilized MTS doses of 10, 15, 20, and 30 mg in children aged 6 to 12 years, inclusive (N = 305), with a DSM-IV-TR primary diagnosis of attention-deficit/hyperactivity disorder. The study was conducted between January 8, 2007, and August 23, 2007. Subjects wore MTS on their hips for 9 hours per day, alternating sides daily for a total of 7 weeks. Assessments included the Experience of Discomfort scale, Transdermal System Adherence scale, and Dermal Response Scale (DRS; 0 = no irritation, 7 = strong reaction). On-study reevaluations were conducted to characterize DRS scores ≥ 4. Epicutaneous allergy patch testing was conducted for DRS scores ≥ 6, persistent DRS scores ≥ 4, DRS score increase following an assessment of ≥ 4, or DRS scores of 4 or 5 following elective discontinuation.
Results: Approximately half of subjects experienced definite erythema at the patch site that generally dissipated within 24 hours. Four subjects experienced a DRS score of 4 (1%): erythema in 1 subject resolved on study treatment, 2 cases resolved poststudy and subjects tolerated oral MPH, and 1 subject discontinued treatment. The latter subject was referred for patch testing and was diagnosed with allergic contact sensitization to MPH.
Conclusions: Few severe dermal effects were seen with MTS treatment. Dermal reactions were characterized as contact dermatitis and dissipated rapidly. On patch testing, 1 subject (0.3%) manifested sensitization to MPH.
Trial registration: clinicaltrials.gov Identifier: NCT00434213.
Figures

References
-
- Bloom B, Dey AN, Freeman G. Summary health statistics for US children: National Health Interview Survey, 2005. Vital Health Stat 10. 2006;231(231):1–84. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 2000. Text Revision.
-
- Biederman J, Faraone S, Milberger S, et al. Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1996;35(3):343–351. - PubMed
-
- Pliszka S. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921. - PubMed
-
- Bush G, Spencer TJ, Holmes J, et al. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65(1):102–114. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
