Deoxypodophyllotoxin Induces a Th1 Response and Enhances the Antitumor Efficacy of a Dendritic Cell-based Vaccine
- PMID: 21494377
- PMCID: PMC3072678
- DOI: 10.4110/in.2011.11.1.79
Deoxypodophyllotoxin Induces a Th1 Response and Enhances the Antitumor Efficacy of a Dendritic Cell-based Vaccine
Abstract
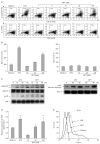
Background: Dendritic cell (DC)-based vaccines are currently being evaluated as a novel strategy for tumor vaccination and immunotherapy. However, inducing long-term regression in established tumor-implanted mice is difficult. Here, we show that deoxypohophyllotoxin (DPT) induces maturation and activation of bone marrow-derived DCs via Toll-like receptor (TLR) 4 activation of MAPK and NF-κB.
Methods: The phenotypic and functional maturation of DPT-treated DCs was assessed by flow cytometric analysis and cytokine production, respectively. DPT-treated DCs was also used for mixed leukocyte reaction to evaluate T cell-priming capacity and for tumor regression against melanoma.
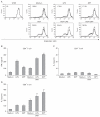
Results: DPT promoted the activation of CD8(+) T cells and the Th1 immune response by inducing IL-12 production in DCs. In a B16F10 melanoma-implanted mouse model, we demonstrated that DPT-treated DCs (DPT-DCs) enhance immune priming and regression of an established tumor in vivo. Furthermore, migration of DPT-DCs to the draining lymph nodes was induced via CCR7 upregulation. Mice that received DPT-DCs displayed enhanced antitumor therapeutic efficacy, which was associated with increased IFN-γ production and induction of cytotoxic T lymphocyte activity.
Conclusion: These findings strongly suggest that the adjuvant effect of DPT in DC vaccination is associated with the polarization of T effector cells toward a Th1 phenotype and provides a potential therapeutic antitumor immunity.
Keywords: CTL activity; DC-based vaccination; Dendritic cells; Deoxypodophyllotoxin; Interleukin-12.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
Figures




Similar articles
-
Phenotypic profile of dendritic and T cells in the lymph node of Balb/C mice with breast cancer submitted to dendritic cells immunotherapy.Immunol Lett. 2016 Sep;177:25-37. doi: 10.1016/j.imlet.2016.07.009. Epub 2016 Jul 14. Immunol Lett. 2016. PMID: 27423825
-
Threonyl-tRNA Synthetase Promotes T Helper Type 1 Cell Responses by Inducing Dendritic Cell Maturation and IL-12 Production via an NF-κB Pathway.Front Immunol. 2020 Oct 14;11:571959. doi: 10.3389/fimmu.2020.571959. eCollection 2020. Front Immunol. 2020. PMID: 33178197 Free PMC article.
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
-
A short hairpin RNA-based adjuvant targeting NF-κB repressor IκBα promotes migration of dermal dendritic cells to draining lymph nodes and antitumor CTL responses induced by DNA vaccination.Vaccine. 2017 Jul 24;35(33):4148-4154. doi: 10.1016/j.vaccine.2017.06.041. Epub 2017 Jun 27. Vaccine. 2017. PMID: 28666759
-
Plant-derived polysaccharides activate dendritic cell-based anti-cancer immunity.Cytotechnology. 2018 Aug;70(4):1097-1110. doi: 10.1007/s10616-018-0202-z. Epub 2018 Mar 19. Cytotechnology. 2018. PMID: 29556897 Free PMC article. Review.
Cited by
-
Nanoparticle-based vaccine delivery for cancer immunotherapy.Immune Netw. 2013 Oct;13(5):177-83. doi: 10.4110/in.2013.13.5.177. Epub 2013 Oct 26. Immune Netw. 2013. PMID: 24198742 Free PMC article. Review.
References
-
- Tobiásová Z, Pospísilová D, Miller AM, Minárik I, Sochorová K, Spísek R, Rob L, Bartůnková J. In vitro assessment of dendritic cells pulsed with apoptotic tumor cells as a vaccine for ovarian cancer patients. Clin Immunol. 2007;122:18–27. - PubMed
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
-
- Morisaki T, Matsumoto K, Onishi H, Kuroki H, Baba E, Tasaki A, Kubo M, Nakamura M, Inaba S, Yamaguchi K, Tanaka M, Katano M. Dendritic cell-based combined immunotherapy with autologous tumor-pulsed dendritic cell vaccine and activated T cells for cancer patients: rationale, current progress, and perspectives. Hum Cell. 2003;16:175–182. - PubMed
-
- Rock KL, Clark K. Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHC class I presentation pathway. J Immunol. 1996;156:3721–3726. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

