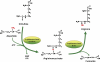Argininosuccinate synthase: at the center of arginine metabolism
- PMID: 21494411
- PMCID: PMC3074183
Argininosuccinate synthase: at the center of arginine metabolism
Abstract
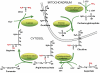
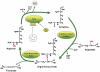
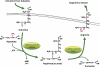
The levels of L-arginine, a cationic, semi-essential amino acid, are often controlled within a cell at the level of local availability through biosynthesis. The importance of this temporal and spatial control of cellular L-arginine is highlighted by the tissue specific roles of argininosuccinate synthase (argininosuccinate synthetase) (EC 6.3.4.5), as the rate-limiting step in the conversion of L-citrulline to L-arginine. Since its discovery, the function of argininosuccinate synthase has been linked almost exclusively to hepatic urea production despite the fact that alternative pathways involving argininosuccinate synthase were defined, such as its role in providing arginine for creatine and for polyamine biosynthesis. However, it was the discovery of nitric oxide that meaningfully extended our understanding of the metabolic importance of non-hepatic argininosuccinate synthase. Indeed, our knowledge of the number of tissues that manage distinct pools of arginine under the control of argininosuccinate synthase has expanded significantly.
Keywords: Argininosuccinate synthase; arginase; arginine metabolism; arginine recycling; nitric oxide; urea cycle.
Figures
References
-
- Morris SM., Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. - PubMed
-
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. - PubMed
-
- Flam BR, Hartmann PJ, Harrell-Booth M, Solo-monson LP, Eichler DC. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide. 2001;5:187–197. - PubMed
-
- Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, Iwakiri Y, Sessa WC. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J Biol Chem. 2004;279:30349–30357. - PubMed
-
- Cohen NS, Kuda A. Argininosuccinate synthetase and argininosuccinate lyase are localized around mitochondria: an immunocytochemical study. J Cell Biochem. 1996;60:334–340. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources