Catalytic, asymmetric reactions of ketenes and ketene enolates
- PMID: 21494417
- PMCID: PMC3074489
- DOI: 10.1016/j.tet.2009.05.079
Catalytic, asymmetric reactions of ketenes and ketene enolates
Figures



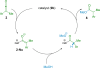
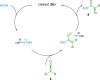
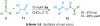
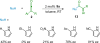
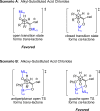
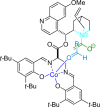
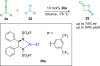
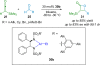



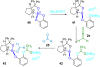
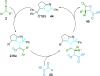


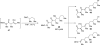

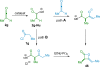

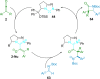
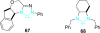
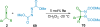

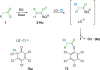
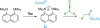
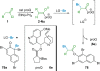
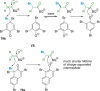
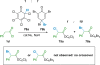

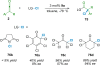
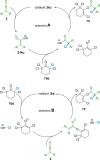
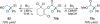

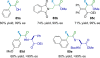
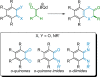
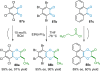
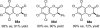
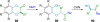
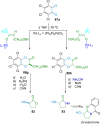
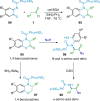
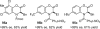
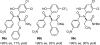


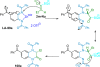

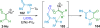

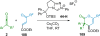
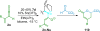
References
-
- Staudinger H. Chem. Ber. 1905;38:1735–1739.
-
-
For the history of ketenes and reviews, see: Tidwell TT. Ketenes. John Wiley & Sons; Hoboken, NJ: 2006. ; Tidwell TT. Eur. J. Org. Chem. 2006:563–576.; Tidwell TT. Angew. Chem., Int. Ed. 2005;44:5778–5785.; Temperley CM. Ketenes, their Cumulene Analogues, and their S, Se, and Te Analogues. In: Katritzky AR, Taylor RJK, editors. Comprehensive Organic Functional Group Transformations II. vol. 3. Elsevier; Amsterdam: 2005. pp. 573–603.; Miller R, Abaecherli C, Said A. Ketenes. 6th ed. Vol. A 15. Ullman's Encyclopedia of Industrial Chemistry; 2002. ; Orr RK, Calter MA. Tetrahedron. 2003;59:3545–3565.; Hyatt JA, Raynolds PW. Org. React. 1994;45:159–646.
-
-
-
For a more involved discussion and leading references, see Ref. 2a.
-
-
- Moore HW, Wilbur DS. J. Org. Chem. 1980;45:4483–4491.
-
- Kresze G, Runge W, Ruch E. Justus Liebigs Ann. Chem. 1972;756:112–127.
Grants and funding
LinkOut - more resources
Full Text Sources
