An efficient method for generating poxvirus recombinants in the absence of selection
- PMID: 21494427
- PMCID: PMC3075091
- DOI: 10.3390/v3030217
An efficient method for generating poxvirus recombinants in the absence of selection
Abstract
The use of selectable markers (ecogpt) and selection pressures to aid in detection of poxvirus (Vaccinia, VV) recombinants has been implicated in the unintended introduction of second site mutations. We have reinvestigated the use of the helper virus system described by Scheiflinger et al. and adapted by Yao and Evans which produces recombinants at a high frequency in the absence of any selection, at a rate of 6–100%. Our system uses fowlpox virus (FPV) as the infectious helper virus which in infected cells provides the enzymatic apparatus for transcription and replication of a purified, transfected VV genome and for recombination with a second transfected PCR generated DNA fragment. To optimize the system, a PCR DNA fragment was generated that contained poxvirus promoter driven gfp and lacZ genes inserted within the coding sequences of the viral thymidine kinase gene. This PCR fragment was co-transfected together with VV genomic DNA. Recombinant VV was identified by plaquing the mixture on cells non-permissive for FPV and selection of green fluorescent or LacZ positive recombinant vaccinia plaques. The system was optimized using FPV permissive cells (CEF) and non-permissive cells (A549, CV-1) for both the initial infection/transfection and the subsequent selection. Up to 70% of the progeny vaccinia virus contained the gfp/LacZ insertion. In order to test for the presence of FPV/VV intertypic recombinants or other unintended mutations, recombinant wtVV (RwtVV) was regenerated from the gfp/LacZ viruses and evaluated by RFLP analysis and pathogenesis in animals. While all RwtVVs were viable in cell culture, in many of the RwtVV isolates, RFLP differences were noted and while some recombinant viruses exhibited wild type behavior in mice, a wide range of virulence indicative of unintended changes suggests that mutants created by “rescue” systems require careful analysis particularly before use for in vivo studies employing animal models.
Keywords: fowlpox virus; helper virus; poxvirus; recombinant; vaccinia virus.
Figures

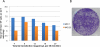

References
-
- Pfleiderer M, Falkner FG, Dorner F. A novel vaccinia virus expression system allowing construction of recombinants without the need for selection markers, plasmids and bacterial hosts. J. Gen. Virol. 1995;76:2957–2962. - PubMed
-
- Timiryasova TM, Chen B, Fodor N, Fodor I. Construction of recombinant vaccinia viruses using PUV-inactivated virus as a helper. Biotechniques. 2001;31:534, 536, 538–540. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
