Production of IFN-β during Listeria monocytogenes infection is restricted to monocyte/macrophage lineage
- PMID: 21494554
- PMCID: PMC3073975
- DOI: 10.1371/journal.pone.0018543
Production of IFN-β during Listeria monocytogenes infection is restricted to monocyte/macrophage lineage
Abstract
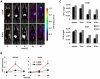
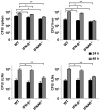
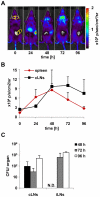
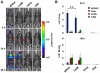
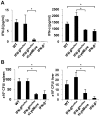
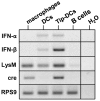
The family of type I interferons (IFN), which consists of several IFN-α and one IFN-β, are produced not only after stimulation by viruses, but also after infection with non-viral pathogens. In the course of bacterial infections, these cytokines could be beneficial or detrimental. IFN-β is the primary member of type I IFN that initiates a cascade of IFN-α production. Here we addressed the question which cells are responsible for IFN-β expression after infection with the intracellular pathogen Listeria monocytogenes by using a genetic approach. By means of newly established reporter mice, maximum of IFN-β expression was observed at 24 hours post infection in spleen and, surprisingly, 48 hours post infection in colonized cervical and inguinal lymph nodes. Colonization of lymph nodes was independent of the type I IFN signaling, as well as bacterial dose and strain. Using cell specific reporter function and conditional deletions we could define cells expressing LysM as the major IFN-β producers, with cells formerly defined as Tip-DCs being the highest. Neutrophilic granulocytes, dendritic cells and plasmacytoid dendritic cells did not significantly contribute to type I IFN production.
Conflict of interest statement
Figures
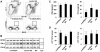
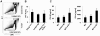
Similar articles
-
Type I IFN Does Not Promote Susceptibility to Foodborne Listeria monocytogenes.J Immunol. 2016 Apr 1;196(7):3109-16. doi: 10.4049/jimmunol.1502192. Epub 2016 Feb 19. J Immunol. 2016. PMID: 26895837 Free PMC article.
-
Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes.PLoS Pathog. 2009 Mar;5(3):e1000355. doi: 10.1371/journal.ppat.1000355. Epub 2009 Mar 27. PLoS Pathog. 2009. PMID: 19325882 Free PMC article.
-
A fluorescence reporter model defines "Tip-DCs" as the cellular source of interferon β in murine listeriosis.PLoS One. 2010 Dec 16;5(12):e15567. doi: 10.1371/journal.pone.0015567. PLoS One. 2010. PMID: 21179567 Free PMC article.
-
Dendritic cells in Listeria monocytogenes infection.Adv Immunol. 2012;113:33-49. doi: 10.1016/B978-0-12-394590-7.00006-3. Adv Immunol. 2012. PMID: 22244578 Review.
-
Monocyte-mediated immune defense against murine Listeria monocytogenes infection.Adv Immunol. 2012;113:119-34. doi: 10.1016/B978-0-12-394590-7.00003-8. Adv Immunol. 2012. PMID: 22244581 Free PMC article. Review.
Cited by
-
Upon intranasal vesicular stomatitis virus infection, astrocytes in the olfactory bulb are important interferon Beta producers that protect from lethal encephalitis.J Virol. 2015 Mar;89(5):2731-8. doi: 10.1128/JVI.02044-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540366 Free PMC article.
-
Herpes virus entry mediator licenses Listeria infection induced immunopathology through control of type I interferon.Sci Rep. 2015 Aug 6;5:12954. doi: 10.1038/srep12954. Sci Rep. 2015. PMID: 26245828 Free PMC article.
-
Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver's Seat.Front Immunol. 2019 Apr 12;10:778. doi: 10.3389/fimmu.2019.00778. eCollection 2019. Front Immunol. 2019. PMID: 31031767 Free PMC article. Review.
-
Monocyte-mediated defense against bacteria, fungi, and parasites.Semin Immunol. 2015 Dec;27(6):397-409. doi: 10.1016/j.smim.2016.03.014. Epub 2016 Mar 25. Semin Immunol. 2015. PMID: 27021645 Free PMC article. Review.
-
Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice.J Virol. 2012 Oct;86(20):11223-30. doi: 10.1128/JVI.01093-12. Epub 2012 Aug 8. J Virol. 2012. PMID: 22875966 Free PMC article.
References
-
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. By A. Isaacs and J. Lindenmann, 1957. J Interferon Res. 1987;7:429–438. - PubMed
-
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. - PubMed
-
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. - PubMed
-
- Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2:378–386. - PubMed
-
- Samuelsson CV, Lienenklaus S, Muller PP, Zawatzky R, Hauser H, et al. Transformation of mouse fibroblasts alters the induction pattern of type I IFNs after virus infection. Biochem Biophys Res Commun. 2005;335:584–589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

