Rgs2 mediates pro-angiogenic function of myeloid derived suppressor cells in the tumor microenvironment via upregulation of MCP-1
- PMID: 21494556
- PMCID: PMC3073977
- DOI: 10.1371/journal.pone.0018534
Rgs2 mediates pro-angiogenic function of myeloid derived suppressor cells in the tumor microenvironment via upregulation of MCP-1
Abstract
Background: Tumor growth is intimately linked with stromal interactions. Myeloid derived suppressor cells (MDSCs) are dramatically elevated in cancer patients and tumor bearing mice. MDSCs modulate the tumor microenvironment through attenuating host immune response and increasing vascularization.
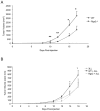
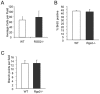
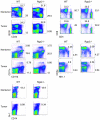
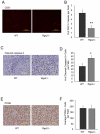
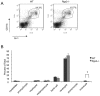
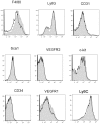
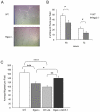
Methodology/principal findings: In searching for molecular mediators responsible for pro-tumor functions, we found that regulator of G protein signaling-2 (Rgs2) is highly increased in tumor-derived MDSCs compared to control MDSCs. We further demonstrate that hypoxia, a common feature associated with solid tumors, upregulates the gene expression. Genetic deletion of Rgs2 in mice resulted in a significant retardation of tumor growth, and the tumors exhibit decreased vascular density and increased cell death. Interestingly, deletion of Rgs2 in MDSCs completely abolished their tumor promoting function, suggesting that Rgs2 signaling in MDSCs is responsible for the tumor promoting function. Cytokine array profiling identified that Rgs2-/- tumor MDSCs produce less MCP-1, leading to decreased angiogenesis, which could be restored with addition of recombinant MCP-1.
Conclusion: Our data reveal Rgs2 as a critical regulator of the pro-angiogenic function of MDSCs in the tumor microenvironment, through regulating MCP-1 production.
Conflict of interest statement
Figures


References
-
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. - PubMed
-
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. - PubMed
-
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. - PubMed
-
- Shojaei F, Wu X, Zhong C, Yu L, Liang XH, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA108856/CA/NCI NIH HHS/United States
- T32HL069765/HL/NHLBI NIH HHS/United States
- R01 NS045888/NS/NINDS NIH HHS/United States
- R01 HL054977/HL/NHLBI NIH HHS/United States
- R21 AI042284/AI/NIAID NIH HHS/United States
- T32 HL069765/HL/NHLBI NIH HHS/United States
- HL054977/HL/NHLBI NIH HHS/United States
- R01 AI061721/AI/NIAID NIH HHS/United States
- T32 CA009582/CA/NCI NIH HHS/United States
- T32CA009582/CA/NCI NIH HHS/United States
- R01 AR053718/AR/NIAMS NIH HHS/United States
- NS45888/NS/NINDS NIH HHS/United States
- AI061721/AI/NIAID NIH HHS/United States
- R01 AI042284/AI/NIAID NIH HHS/United States
- AR053718/AR/NIAMS NIH HHS/United States
- AI042284/AI/NIAID NIH HHS/United States
- CA108856/CA/NCI NIH HHS/United States
- T32 AI007611/AI/NIAID NIH HHS/United States
- T32AI007611/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

