Sequential and coordinated actions of c-Myc and N-Myc control appendicular skeletal development
- PMID: 21494559
- PMCID: PMC3073980
- DOI: 10.1371/journal.pone.0018795
Sequential and coordinated actions of c-Myc and N-Myc control appendicular skeletal development
Abstract
Background: During limb development, chondrocytes and osteoblasts emerge from condensations of limb bud mesenchyme. These cells then proliferate and differentiate in separate but adjacent compartments and function cooperatively to promote bone growth through the process of endochondral ossification. While many aspects of limb skeletal formation are understood, little is known about the mechanisms that link the development of undifferentiated limb bud mesenchyme with formation of the precartilaginous condensation and subsequent proliferative expansion of chondrocyte and osteoblast lineages. The aim of this study was to gain insight into these processes by examining the roles of c-Myc and N-Myc in morphogenesis of the limb skeleton.
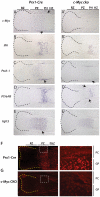
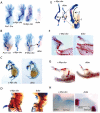
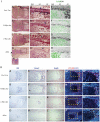
Methodology/principal findings: To investigate c-Myc function in skeletal development, we characterized mice in which floxed c-Myc alleles were deleted in undifferentiated limb bud mesenchyme with Prx1-Cre, in chondro-osteoprogenitors with Sox9-Cre and in osteoblasts with Osx1-Cre. We show that c-Myc promotes the proliferative expansion of both chondrocytes and osteoblasts and as a consequence controls the process of endochondral growth and ossification and determines bone size. The control of proliferation by c-Myc was related to its effects on global gene transcription, as phosphorylation of the C-Terminal Domain (pCTD) of RNA Polymerase II, a marker of general transcription initiation, was tightly coupled to cell proliferation of growth plate chondrocytes where c-Myc is expressed and severely downregulated in the absence of c-Myc. Finally, we show that combined deletion of N-Myc and c-Myc in early limb bud mesenchyme gives rise to a severely hypoplastic limb skeleton that exhibits features characteristic of individual c-Myc and N-Myc mutants.
Conclusions/significance: Our results show that N-Myc and c-Myc act sequentially during limb development to coordinate the expansion of key progenitor populations responsible for forming the limb skeleton.
Conflict of interest statement
Figures
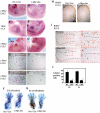
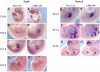
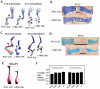
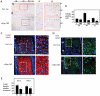


References
-
- Niswander L. Pattern formation: old models out on a limb. Nat. Rev. . Genet. 2003;4:133–143. - PubMed
-
- Tabin C, Wolpert L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007;21:1433–1442. - PubMed
-
- Thorogood PV, Hinchliffe JR. An analysis of the condensation process during chondrogenesis in the embryonic chick hind limb. J Embryol Exp Morphol. 1975;33:581–606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

