Ptenb mediates gastrulation cell movements via Cdc42/AKT1 in zebrafish
- PMID: 21494560
- PMCID: PMC3073981
- DOI: 10.1371/journal.pone.0018702
Ptenb mediates gastrulation cell movements via Cdc42/AKT1 in zebrafish
Abstract
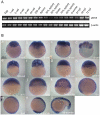
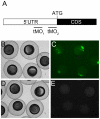
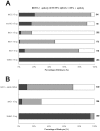
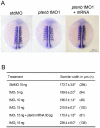
Phosphatidylinositol 3-kinase (PI3 kinase) mediates gastrulation cell migration in zebrafish via its regulation of PIP(2)/PIP(3) balance. Although PI3 kinase counter enzyme PTEN has also been reported to be essential for gastrulation, its role in zebrafish gastrulation has been controversial due to the lack of gastrulation defects in pten-null mutants. To clarify this issue, we knocked down a pten isoform, ptenb by using anti-sense morpholino oligos (MOs) in zebrafish embryos and found that ptenb MOs inhibit convergent extension by affecting cell motility and protrusion during gastrulation. The ptenb MO-induced convergence defect could be rescued by a PI3-kinase inhibitor, LY294002 and by overexpressing dominant negative Cdc42. Overexpression of human constitutively active akt1 showed similar convergent extension defects in zebrafish embryos. We also observed a clear enhancement of actin polymerization in ptenb morphants under cofocal microscopy and in actin polymerization assay. These results suggest that Ptenb by antagonizing PI3 kinase and its downstream Akt1 and Cdc42 to regulate actin polymerization that is critical for proper cell motility and migration control during gastrulation in zebrafish.
Conflict of interest statement
Figures






References
-
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. - PubMed
-
- Solnica-Krezel L, Cooper MS. Cellular and genetic mechanisms of convergence and extension. Results Probl Cell Differ. 2002;40:136–165. - PubMed
-
- Schier AF. Axis formation and patterning in zebrafish. Curr Opin Genet Dev. 2001;11:393–404. - PubMed
-
- Warga RM, Kimmel CB. Cell movements during epiboly and gastrulation in zebrafish. Development. 1990;108:569–580. - PubMed
-
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

