Regulatory response to carbon starvation in Caulobacter crescentus
- PMID: 21494595
- PMCID: PMC3073932
- DOI: 10.1371/journal.pone.0018179
Regulatory response to carbon starvation in Caulobacter crescentus
Abstract
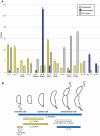
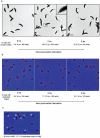
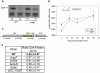
Bacteria adapt to shifts from rapid to slow growth, and have developed strategies for long-term survival during prolonged starvation and stress conditions. We report the regulatory response of C. crescentus to carbon starvation, based on combined high-throughput proteome and transcriptome analyses. Our results identify cell cycle changes in gene expression in response to carbon starvation that involve the prominent role of the FixK FNR/CAP family transcription factor and the CtrA cell cycle regulator. Notably, the SigT ECF sigma factor mediates the carbon starvation-induced degradation of CtrA, while activating a core set of general starvation-stress genes that respond to carbon starvation, osmotic stress, and exposure to heavy metals. Comparison of the response of swarmer cells and stalked cells to carbon starvation revealed four groups of genes that exhibit different expression profiles. Also, cell pole morphogenesis and initiation of chromosome replication normally occurring at the swarmer-to-stalked cell transition are uncoupled in carbon-starved cells.
Conflict of interest statement
Figures


References
-
- Goley ED, Iniesta AA, Shapiro L. Cell cycle regulation in Caulobacter: location, location, location. J Cell Sci. 2007;120:3501–3507. - PubMed
-
- Holtzendorff J, Reinhardt J, Viollier PH. Cell cycle control by oscillating regulatory proteins in Caulobacter crescentus. Bioessays. 2006;28:355–361. - PubMed
-
- Jacobs-Wagner C. Regulatory proteins with a sense of direction: cell cycle signalling network in Caulobacter. Mol Microbiol. 2004;51:7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

