Evidence for polymicrobic flora translocating in peripheral blood of HIV-infected patients with poor immune response to antiretroviral therapy
- PMID: 21494598
- PMCID: PMC3073938
- DOI: 10.1371/journal.pone.0018580
Evidence for polymicrobic flora translocating in peripheral blood of HIV-infected patients with poor immune response to antiretroviral therapy
Abstract
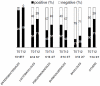
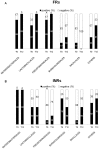
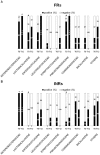
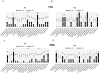
In advanced HIV infection, the homeostatic balance between gastrointestinal indigenous bacteria and gut immunity fails and microbes are able to overcome the intestinal barrier and gain the systemic circulation. Because microbial translocation is not fully controlled by antiviral therapy and is associated with inefficient CD4+ reconstitution, we investigated the profile of translocating bacteria in peripheral blood of 44 HIV-infected patients starting therapy with advanced CD4+ T-lymphopenia and displaying poor CD4+ recovery on virologically suppressive HAART. According to CD4+ reconstitution at 12-months HAART, patients were considered Partial Immunological Responders, PIRs (CD4+≥250/µl, n = 29) and Immunological non Responders, INRs (CD4+<200/µl, n = 15)). We show that PIRs and INRs present similarly elevated plasma levels of lipopolysaccharide (LPS) and its ligand sCD14 that were not lowered by virologically suppressive therapy. Bacterial 16S rRNA gene amplification and sequencing resulted in a highly polymicrobic peripheral blood microbiota both prior and after 12-month HAART. Several differences in bacterial composition were shown between patients' groups, mainly the lack of probiotic Lactobacillaceae both prior and after therapy in INRs. Failure to control microbial translocation on HAART is associated with a polymicrobic flora circulating in peripheral blood that is not substantially modified by therapy.
Conflict of interest statement
Figures
References
-
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. - PubMed
-
- Ferri E, Novati S, Casiraghi M, Sambri V, Genco F, et al. Plasma levels of bacterial DNA in HIV infection: the limits of quantitative polymerase chain reaction. J Infect Dis. 202:176–177; author reply 178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

