An analysis of the cross-reactivity of autoantibodies to GAD65 and GAD67 in diabetes
- PMID: 21494613
- PMCID: PMC3072979
- DOI: 10.1371/journal.pone.0018411
An analysis of the cross-reactivity of autoantibodies to GAD65 and GAD67 in diabetes
Abstract
Background: Autoantibodies to GAD65 (anti-GAD65) are present in the sera of 70-80% of patients with type 1 diabetes (T1D), but antibodies to the structurally similar 67 kDa isoform GAD67 are rare. Antibodies to GAD67 may represent a cross-reactive population of anti-GAD65, but this has not been formally tested.
Methodology/principal findings: In this study we examined the frequency, levels and affinity of anti-GAD67 in diabetes sera that contained anti-GAD65, and compared the specificity of GAD65 and GAD67 reactivity. Anti-GAD65 and anti-GAD67 were measured by radioimmunoprecipitation (RIP) using (125)I labeled recombinant GAD65 and GAD67. For each antibody population, the specificity of the binding was measured by incubation with 100-fold excess of unlabeled GAD in homologous and heterologous inhibition assays, and the affinity of binding with GAD65 and GAD67 was measured in selected sera. Sera were also tested for reactivity to GAD65 and GAD67 by immunoblotting. Of the 85 sera that contained antibodies to GAD65, 28 contained anti-GAD67 measured by RIP. Inhibition with unlabeled GAD65 substantially or completely reduced antibody reactivity with both (125)I GAD65 and with (125)I GAD67. In contrast, unlabeled GAD67 reduced autoantibody reactivity with (125)I GAD67 but not with (125)I GAD65. Both populations of antibodies were of high affinity (>10(10) l/mol).
Conclusions: Our findings show that autoantibodies to GAD67 represent a minor population of anti-GAD65 that are reactive with a cross-reactive epitope found also on GAD67. Experimental results confirm that GAD65 is the major autoantigen in T1D, and that GAD67 per se has very low immunogenicity. We discuss our findings in light of the known similarities between the structures of the GAD isoforms, in particular the location of a minor cross-reactive epitope that could be induced by epitope spreading.
Conflict of interest statement
Figures

 , (□) and (▴) represent three different sera samples.
, (□) and (▴) represent three different sera samples.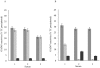
References
-
- Lernmark A. Glutamic acid decarboxylase–gene to antigen to disease. J Intern Med. 1996;240:259–277. - PubMed
-
- Tuomi T, Carlsson A, Li H, Isomaa B, Miettinen A, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48:150–157. - PubMed
-
- Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med. 1990;322:1555–1560. - PubMed
-
- Solimena M, De Camilli P. Autoimmunity to glutamic acid decarboxylase (GAD) in Stiff-Man syndrome and insulin-dependent diabetes mellitus. Trends Neurosci. 1991;14:452–457. - PubMed
-
- Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

