Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury
- PMID: 21494626
- PMCID: PMC3072992
- DOI: 10.1371/journal.pone.0018683
Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury
Abstract
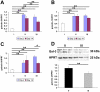
Galectin-3 is a β-galactoside binding lectin with roles in diverse processes including proliferation, apoptosis, inflammation and fibrosis which are dependent on different domains of the molecule and subcellular distribution. Although galectin-3 is known to be upregulated in acute kidney injury, the relative importance of its different domains and functions are poorly understood in the underlying pathogenesis. Therefore we experimentally modulated galectin-3 in folic acid (FA)-induced acute kidney injury utilising modified citrus pectin (MCP), a derivative of pectin which can bind to the galectin-3 carbohydrate recognition domain thereby predominantly antagonising functions linked to this role. Mice were pre-treated with normal or 1% MCP-supplemented drinking water one week before FA injection. During the initial injury phase, all FA-treated mice lost weight whilst their kidneys enlarged secondary to the renal insult; these gross changes were significantly lessened in the MCP group but this was not associated with significant changes in galectin-3 expression. At a histological level, MCP clearly reduced renal cell proliferation but did not affect apoptosis. Later, during the recovery phase at two weeks, MCP-treated mice demonstrated reduced galectin-3 in association with decreased renal fibrosis, macrophages, pro-inflammatory cytokine expression and apoptosis. Other renal galectins, galectin-1 and -9, were unchanged. Our data indicates that MCP is protective in experimental nephropathy with modulation of early proliferation and later galectin-3 expression, apoptosis and fibrosis. This raises the possibility that MCP may be a novel strategy to reduce renal injury in the long term, perhaps via carbohydrate binding-related functions of galectin-3.
Conflict of interest statement
Figures






Similar articles
-
Galectin 3 inhibition attenuates renal injury progression in cisplatin-induced nephrotoxicity.Biosci Rep. 2018 Dec 18;38(6):BSR20181803. doi: 10.1042/BSR20181803. Print 2018 Dec 21. Biosci Rep. 2018. PMID: 30455396 Free PMC article.
-
Galectin-3 pharmacological inhibition attenuates early renal damage in spontaneously hypertensive rats.J Hypertens. 2018 Feb;36(2):368-376. doi: 10.1097/HJH.0000000000001545. J Hypertens. 2018. PMID: 28858976
-
Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells.Can J Physiol Pharmacol. 2016 May;94(5):554-62. doi: 10.1139/cjpp-2015-0284. Epub 2015 Dec 16. Can J Physiol Pharmacol. 2016. PMID: 27010252
-
Pleiotropic Effects of Modified Citrus Pectin.Nutrients. 2019 Nov 1;11(11):2619. doi: 10.3390/nu11112619. Nutrients. 2019. PMID: 31683865 Free PMC article. Review.
-
Modified citrus pectin anti-metastatic properties: one bullet, multiple targets.Carbohydr Res. 2009 Sep 28;344(14):1788-91. doi: 10.1016/j.carres.2008.08.038. Epub 2008 Sep 26. Carbohydr Res. 2009. PMID: 19061992 Free PMC article. Review.
Cited by
-
Pharmacological Inhibition of Galectin-3 Ameliorates Diabetes-Associated Cognitive Impairment, Oxidative Stress and Neuroinflammation in vivo and in vitro.J Inflamm Res. 2020 Sep 15;13:533-542. doi: 10.2147/JIR.S273858. eCollection 2020. J Inflamm Res. 2020. PMID: 32982368 Free PMC article.
-
Investigation of antioxidant and anticancer activities of unsaturated oligo-galacturonic acids produced by pectinase of Streptomyces hydrogenans YAM1.Sci Rep. 2021 Apr 19;11(1):8491. doi: 10.1038/s41598-021-87804-9. Sci Rep. 2021. PMID: 33875695 Free PMC article.
-
Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target.Int J Mol Sci. 2022 Mar 14;23(6):3124. doi: 10.3390/ijms23063124. Int J Mol Sci. 2022. PMID: 35328545 Free PMC article. Review.
-
The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement?Biomolecules. 2022 Feb 10;12(2):289. doi: 10.3390/biom12020289. Biomolecules. 2022. PMID: 35204790 Free PMC article. Review.
-
Serum Galectin and Renal Dysfunction in ST-Segment Elevation Myocardial Infarction.Dis Markers. 2016;2016:1549063. doi: 10.1155/2016/1549063. Epub 2016 Feb 15. Dis Markers. 2016. PMID: 26980923 Free PMC article.
References
-
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, et al. Galectins: a family of animal β-galactoside binding lectins. Cell. 1994;76:597–598. - PubMed
-
- Leffler H, Barondes SH. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian β-galactosides. J Biol Chem. 1986;261:10119–10126. - PubMed
-
- Lin HM, Pestell RG, Raz A, Kim HR. Galectin-3 enhances cyclin D1 promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells. Oncogene. 2002;21:8001–8010. - PubMed
-
- Davidson PJ, Davis MJ, Patterson RJ, Ripoche MA, Poirier F, et al. Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology. 2002;12:329–337. - PubMed
-
- Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;19:263–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

