Development and validation of decision rules to guide frequency of monitoring CD4 cell count in HIV-1 infection before starting antiretroviral therapy
- PMID: 21494630
- PMCID: PMC3072996
- DOI: 10.1371/journal.pone.0018578
Development and validation of decision rules to guide frequency of monitoring CD4 cell count in HIV-1 infection before starting antiretroviral therapy
Abstract
Background: Although CD4 cell count monitoring is used to decide when to start antiretroviral therapy in patients with HIV-1 infection, there are no evidence-based recommendations regarding its optimal frequency. It is common practice to monitor every 3 to 6 months, often coupled with viral load monitoring. We developed rules to guide frequency of CD4 cell count monitoring in HIV infection before starting antiretroviral therapy, which we validated retrospectively in patients from the Swiss HIV Cohort Study.
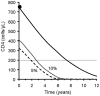
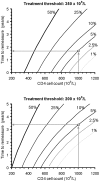
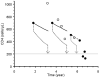
Methodology/principal findings: We built up two prediction rules ("Snap-shot rule" for a single sample and "Track-shot rule" for multiple determinations) based on a systematic review of published longitudinal analyses of CD4 cell count trajectories. We applied the rules in 2608 untreated patients to classify their 18 061 CD4 counts as either justifiable or superfluous, according to their prior ≥5% or <5% chance of meeting predetermined thresholds for starting treatment. The percentage of measurements that both rules falsely deemed superfluous never exceeded 5%. Superfluous CD4 determinations represented 4%, 11%, and 39% of all actual determinations for treatment thresholds of 500, 350, and 200×10(6)/L, respectively. The Track-shot rule was only marginally superior to the Snap-shot rule. Both rules lose usefulness for CD4 counts coming near to treatment threshold.
Conclusions/significance: Frequent CD4 count monitoring of patients with CD4 counts well above the threshold for initiating therapy is unlikely to identify patients who require therapy. It appears sufficient to measure CD4 cell count 1 year after a count >650 for a threshold of 200, >900 for 350, or >1150 for 500×10(6)/L, respectively. When CD4 counts fall below these limits, increased monitoring frequency becomes advisable. These rules offer guidance for efficient CD4 monitoring, particularly in resource-limited settings.
Conflict of interest statement
Figures
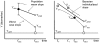
Similar articles
-
Diagnostic accuracy of CD4 cell count increase for virologic response after initiating highly active antiretroviral therapy.AIDS. 2006 Aug 1;20(12):1613-9. doi: 10.1097/01.aids.0000238407.00874.dc. AIDS. 2006. PMID: 16868442
-
Optimal frequency of CD4 cell count and HIV RNA monitoring prior to initiation of antiretroviral therapy in HIV-infected patients.Antivir Ther. 2005;10(1):41-52. Antivir Ther. 2005. PMID: 15751762
-
Safety of monitoring antiretroviral therapy response in HIV-1 infection using CD4+ T cell count at long-term intervals.Cad Saude Publica. 2018 Oct 22;34(10):e00009618. doi: 10.1590/0102-311X00009618. Cad Saude Publica. 2018. PMID: 30365742
-
[Recommendations from the GESIDA/Spanish AIDS Plan regarding antiretroviral treatment in adults with human immunodeficiency virus infection (update February 2009)].Enferm Infecc Microbiol Clin. 2009 Apr;27(4):222-35. doi: 10.1016/j.eimc.2008.11.002. Epub 2009 Feb 26. Enferm Infecc Microbiol Clin. 2009. PMID: 19246124 Spanish.
-
Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel.JAMA. 2008 Aug 6;300(5):555-70. doi: 10.1001/jama.300.5.555. JAMA. 2008. PMID: 18677028
Cited by
-
Should malaria treatment be guided by a point of care rapid test? A threshold approach to malaria management in rural Burkina Faso.PLoS One. 2013;8(3):e58019. doi: 10.1371/journal.pone.0058019. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23472129 Free PMC article.
-
Factors affecting timing of antiretroviral treatment initiation based on monitoring CD4 counts.J Acquir Immune Defic Syndr. 2012 Nov 1;61(3):326-33. doi: 10.1097/QAI.0b013e31826be75e. J Acquir Immune Defic Syndr. 2012. PMID: 22878419 Free PMC article.
-
Adherence to highly active antiretroviral treatment in HIV-infected Rwandan women.PLoS One. 2011;6(11):e27832. doi: 10.1371/journal.pone.0027832. Epub 2011 Nov 17. PLoS One. 2011. PMID: 22114706 Free PMC article.
-
Reply.Med J Armed Forces India. 2014 Jul;70(3):301-2. doi: 10.1016/j.mjafi.2014.06.013. Med J Armed Forces India. 2014. PMID: 25378795 Free PMC article. No abstract available.
-
The basis for monitoring strategies in clinical guidelines: a case study of prostate-specific antigen for monitoring in prostate cancer.CMAJ. 2012 Feb 7;184(2):169-77. doi: 10.1503/cmaj.110600. Epub 2011 Dec 12. CMAJ. 2012. PMID: 22158408 Free PMC article.
References
-
- Thompson MA, Aberg JA, Cahn P, Montaner JSG, Rizzardini G, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA Panel. JAMA. 2010;304:321–333. - PubMed
-
- Hammer SM Clinical practice. Management of newly diagnosed HIV infection. N Engl J Med. 2005;353:1702–1710. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

