Identification of single nucleotide polymorphisms associated with hyperproduction of alpha-toxin in Staphylococcus aureus
- PMID: 21494631
- PMCID: PMC3072997
- DOI: 10.1371/journal.pone.0018428
Identification of single nucleotide polymorphisms associated with hyperproduction of alpha-toxin in Staphylococcus aureus
Abstract
The virulence factor α-toxin (hla) is needed by Staphylococcus aureus in order to cause infections in both animals and humans. Although the complicated regulation of hla expression has been well studied in human S. aureus isolates, the mechanisms of of hla regulation in bovine S. aureus isolates remain undefined. In this study, we found that many bovine S. aureus isolates, including the RF122 strain, generate dramatic amounts of α-toxin in vitro compared with human clinical S. aureus isolates, including MRSA WCUH29 and MRSA USA300. To elucidate potential regulatory mechanisms, we analyzed the hla promoter regions and identified predominant single nucleotide polymorphisms (SNPs) at positions -376, -483, and -484 from the start codon in α-toxin hyper-producing isolates. Using site-directed mutagenesis and hla promoter-gfp-luxABCDE dual reporter approaches, we demonstrated that the SNPs contribute to the differential control of hla expression among bovine and human S. aureus isolates. Using a DNA affinity assay, gel-shift assays and a null mutant, we identified and revealed that an hla positive regulator, SarZ, contributes to the involvement of the SNPs in mediating hla expression. In addition, we found that the bovine S. aureus isolate RF122 exhibits higher transcription levels of hla positive regulators, including agrA, saeR, arlR and sarZ, but a lower expression level of hla repressor rot compared to the human S. aureus isolate WCUH29. Our results indicate α-toxin hyperproduction in bovine S. aureus is a multifactorial process, influenced at both the genomic and transcriptional levels. Moreover, the identification of predominant SNPs in the hla promoter region may provide a novel method for genotyping the S. aureus isolates.
Conflict of interest statement
Figures


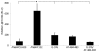

Similar articles
-
Virulence gene profiles: alpha-hemolysin and clonal diversity in Staphylococcus aureus isolates from bovine clinical mastitis in China.BMC Vet Res. 2018 Mar 2;14(1):63. doi: 10.1186/s12917-018-1374-7. BMC Vet Res. 2018. PMID: 29499697 Free PMC article.
-
Identification of predominant SNPs as a novel method for genotyping bovine Staphylococcus aureus isolates.Virulence. 2012 Jan-Feb;3(1):98-102. doi: 10.4161/viru.3.1.18724. Epub 2012 Jan 1. Virulence. 2012. PMID: 22286701
-
Identification, cloning, and initial characterization of rot, a locus encoding a regulator of virulence factor expression in Staphylococcus aureus.J Bacteriol. 2000 Jun;182(11):3197-203. doi: 10.1128/JB.182.11.3197-3203.2000. J Bacteriol. 2000. PMID: 10809700 Free PMC article.
-
Long Noncoding RNA SSR42 Controls Staphylococcus aureus Alpha-Toxin Transcription in Response to Environmental Stimuli.J Bacteriol. 2018 Oct 23;200(22):e00252-18. doi: 10.1128/JB.00252-18. Print 2018 Nov 15. J Bacteriol. 2018. PMID: 30150231 Free PMC article.
-
Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates.Antimicrob Agents Chemother. 1998 Nov;42(11):2817-23. doi: 10.1128/AAC.42.11.2817. Antimicrob Agents Chemother. 1998. PMID: 9797209 Free PMC article.
Cited by
-
High-resolution transcriptome maps reveal strain-specific regulatory features of multiple Campylobacter jejuni isolates.PLoS Genet. 2013 May;9(5):e1003495. doi: 10.1371/journal.pgen.1003495. Epub 2013 May 16. PLoS Genet. 2013. PMID: 23696746 Free PMC article.
-
Host factors determine the evolution of infection with Staphylococcus aureus to gangrenous mastitis in goats.Vet Res. 2018 Jul 25;49(1):72. doi: 10.1186/s13567-018-0564-4. Vet Res. 2018. PMID: 30045763 Free PMC article.
-
Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA).FEMS Immunol Med Microbiol. 2012 Jun;65(1):5-22. doi: 10.1111/j.1574-695X.2012.00937.x. Epub 2012 Mar 5. FEMS Immunol Med Microbiol. 2012. PMID: 22309135 Free PMC article. Review.
-
Virulence gene profiles: alpha-hemolysin and clonal diversity in Staphylococcus aureus isolates from bovine clinical mastitis in China.BMC Vet Res. 2018 Mar 2;14(1):63. doi: 10.1186/s12917-018-1374-7. BMC Vet Res. 2018. PMID: 29499697 Free PMC article.
-
The SaeRS Two-Component System Controls Survival of Staphylococcus aureus in Human Blood through Regulation of Coagulase.Front Cell Infect Microbiol. 2017 May 29;7:204. doi: 10.3389/fcimb.2017.00204. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28611950 Free PMC article.
References
-
- Rice L. Antimicrobial resistance in Gram-positive bacteria. Am J Med. 2006;119:S11–S19. - PubMed
-
- Novick RP. Staphylococcal pathogenesis and pathogenicity factors: genetics and regulation. In: Fischetti V, et al., editors. Gram-positive pathogens. Washington, D.C.: ASM Press; 2006. pp. 496–516.
-
- Foster TJ, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends in Microbiol. 1998;6:484–488. - PubMed
-
- Lowy FD. Staphylococcus aureus Infections. N Engl J Med. 1998;339:520–532. Ballal A, Ray B, Manna AC (2009) sarZ, a sarA Family Gene, Is Transcriptionally Activated by MgrA and Is Involved in the Regulation of Genes Encoding Exoproteins in Staphylococcus aureus. J Bacteriol 191: 1656–1665. - PubMed
-
- Essmann F, Bantel H, Totzke G, Engels IH, Sinha B, et al. Staphylococcus aureus alpha-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 2003;10:1260–1272. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

