Decreased numbers of blood dendritic cells and defective function of regulatory T cells in antineutrophil cytoplasmic antibody-associated vasculitis
- PMID: 21494636
- PMCID: PMC3073002
- DOI: 10.1371/journal.pone.0018734
Decreased numbers of blood dendritic cells and defective function of regulatory T cells in antineutrophil cytoplasmic antibody-associated vasculitis
Abstract
Background: Dendritic cells (DC) and regulatory cells (Treg) play pivotal roles in controlling both normal and autoimmune adaptive immune responses. DC are the main antigen-presenting cells to T cells, and they also control Treg functions. In this study, we examined the frequency and phenotype of DC subsets, and the frequency and function of Treg from patients with ANCA-associated vasculitis (AAV).
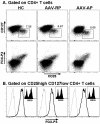
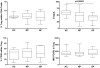
Methodology/principal findings: Blood samples from 19 untreated patients with AAV during flares and before any immunosuppressive treatment were analyzed, along with 15 AAV patients in remission and 18 age-matched healthy controls. DC and Treg numbers, and phenotypes were assessed by flow cytometry, and in vitro suppressive function of Treg was determined by co-culture assay. When compared to healthy volunteers, absolute numbers of conventional and plasmacytoid DC were decreased in AAV patients. During the acute phase this decrease was significantly more pronounced and was associated with an increased DC expression of CD62L. Absolute numbers of Treg (CD4(+)CD25(high)CD127(low/-) Tcells) were moderately decreased in patients. FOXP3 and CD39 were expressed at similar levels on Treg from patients as compared to controls. The suppressive function of Treg from AAV patients was dramatically decreased as compared to controls, and this defect was more pronounced during flares than remission. This Treg functional deficiency occurred in the absence of obvious Th17 deviation.
Conclusion: In conclusion, these data show that AAV flares are associated with both a decrease number and altered phenotype of circulating DC and point to a role for Treg functional deficiency in the pathogenesis of AAV.
Conflict of interest statement
Figures



References
-
- van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–429. - PubMed
-
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. - PubMed
-
- Boomsma MM, Stegeman CA, van der Leij MJ, Oost W, Hermans J, et al. Prediction of relapses in Wegener's granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43:2025–2033. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

