Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells
- PMID: 21494638
- PMCID: PMC3071840
- DOI: 10.1371/journal.pone.0018562
Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells
Abstract
Background: Increased expression of the cyclooxygenase-2 enzyme (COX2) is one of the main characteristics of gastric cancer (GC), which is a leading cause of death in the world, particularly in Asia and South America. Although the Wnt/β-catenin signaling pathway has been involved in the transcriptional activation of the COX2 gene, the precise mechanism modulating this response is still unknown.
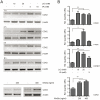
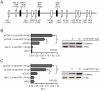
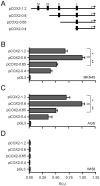
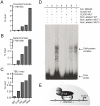
Methodology/principal findings: Here we studied the transcriptional regulation of the COX2 gene in GC cell lines and assessed whether this phenomenon is modulated by Wnt/β-catenin signaling. We first examined the expression of COX2 mRNA in GC cells and found that there is a differential expression pattern consistent with high levels of nuclear-localized β-catenin. Pharmacological treatment with either lithium or valproic acid and molecular induction with purified canonical Wnt3a significantly enhanced COX2 mRNA expression in a dose- and time-dependent manner. Serial deletion of a 1.6 Kbp COX2 promoter fragment and gain- or loss-of-function experiments allowed us to identify a minimal Wnt/β-catenin responsive region consisting of 0.8 Kbp of the COX2 promoter (pCOX2-0.8), which showed maximal response in gene-reporter assays. The activity of this pCOX2-0.8 promoter region was further confirmed by site-directed mutagenesis and DNA-protein binding assays.
Conclusions/significance: We conclude that the pCOX2-0.8 minimal promoter contains a novel functional T-cell factor/lymphoid enhancer factor (TCF/LEF)-response element (TBE Site II; -689/-684) that responds directly to enhanced Wnt/β-catenin signaling and which may be important for the onset/progression of GC.
Conflict of interest statement
Figures


References
-
- Parkin DM. International variation. Oncogene. 2004;23:6329–6340. - PubMed
-
- Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5:121–125. - PubMed
-
- Konturek PC, Konturek SJ, Brzozowski T. Gastric cancer and Helicobacter pylori infection. J Physiol Pharmacol. 2006;57(Suppl 3):51–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

