Antibody responses against xenotropic murine leukemia virus-related virus envelope in a murine model
- PMID: 21494670
- PMCID: PMC3071813
- DOI: 10.1371/journal.pone.0018272
Antibody responses against xenotropic murine leukemia virus-related virus envelope in a murine model
Erratum in
- PLoS One. 2011;6(5). doi:10.1371/annotation/913fdc1e-877e-4c70-ac20-761d2d72400d
Abstract
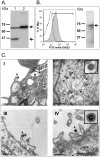
Background: Xenotropic murine leukemia virus-related virus (XMRV) was recently discovered to be the first human gammaretrovirus that is associated with chronic fatigue syndrome and prostate cancer (PC). Although a mechanism for XMRV carcinogenesis is yet to be established, this virus belongs to the family of gammaretroviruses well known for their ability to induce cancer in the infected hosts. Since its original identification XMRV has been detected in several independent investigations; however, at this time significant controversy remains regarding reports of XMRV detection/prevalence in other cohorts and cell type/tissue distribution. The potential risk of human infection, coupled with the lack of knowledge about the basic biology of XMRV, warrants further research, including investigation of adaptive immune responses. To study immunogenicity in vivo, we vaccinated mice with a combination of recombinant vectors expressing codon-optimized sequences of XMRV gag and env genes and virus-like particles (VLP) that had the size and morphology of live infectious XMRV.
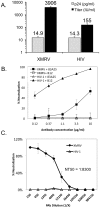
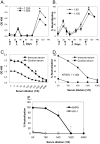
Results: Immunization elicited Env-specific binding and neutralizing antibodies (NAb) against XMRV in mice. The peak titers for ELISA-binding antibodies and NAb were 1:1024 and 1:464, respectively; however, high ELISA-binding and NAb titers were not sustained and persisted for less than three weeks after immunizations.
Conclusions: Vaccine-induced XMRV Env antibody titers were transiently high, but their duration was short. The relatively rapid diminution in antibody levels may in part explain the differing prevalences reported for XMRV in various prostate cancer and chronic fatigue syndrome cohorts. The low level of immunogenicity observed in the present study may be characteristic of a natural XMRV infection in humans.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

