Transglutaminases (TGs) in ocular and periocular tissues: effect of muscarinic agents on TGs in scleral fibroblasts
- PMID: 21494676
- PMCID: PMC3071819
- DOI: 10.1371/journal.pone.0018326
Transglutaminases (TGs) in ocular and periocular tissues: effect of muscarinic agents on TGs in scleral fibroblasts
Abstract
Objective: To investigate the expression of transglutaminases (TGs) in the ocular surface, the eyelid margin and associated glands and to determine effect of muscarinic agents on TGs in scleral fibroblasts (SF).
Materials and methods: Primary SFs cultured from mouse and human sclera were treated with atropine and carbachol for 5 days. Lysed cell RNA was used for real-time PCR, protein was used for Western blot analysis and TG-2 transamidase activity was measured by ELISA. Immunohistochemistry was done to determine the expression of TGases.
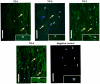
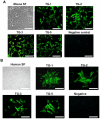
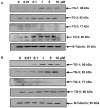
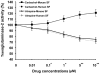
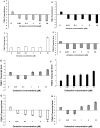
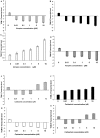
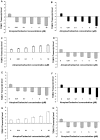
Results: Immunohistochemistry and western blot confirmed the expression of TGs-1, 2, 3 and 5 proteins in cultured SFs and eye tissues. Real time PCR showed TG-1, 2, 5 transcript levels to be down regulated 3 fold (p<0.05) in cultured human and mouse SFs after incubation with atropine and this was reversed by carbachol. However, TG-3 expression was increased with atropine and decreased with carbachol at all concentrations. Atropine abrogated the carbachol-induced activation of SF in a dose-dependent manner. TGs-1, 3, 5 were localized in the entire mouse corneal epithelium, stroma and endothelium but TG-2 was present only in the corneal subepithelium and stroma. All TGs were localized in mouse Meibomian glands however TG-2 had a weak expression.
Conclusions: Our results confirm that TGs-1, 2, 3 and 5 are expressed in human SF and murine ocular tissues, eyelid and associated Meibomian glands. Real-time PCR and Western blot results showed that muscarinic antagonist down-regulates TGs-1, 2 and 5 in both cultured human and mouse SFs and upregulates TG-3. Atropine abrogated the carbachol-induced activation of SF in a dose-dependent manner. These results suggest that manipulation of TGs by way of muscarinic receptor acting drugs may be a plausible method of intervention in wound healing and scleral remodeling.
Conflict of interest statement
Figures



References
-
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. - PubMed
-
- Verderio EAM, Johnson T, Griffin M. Tissue transglutaminase in normal and abnormal wound healing: Review article. Amino Acids. 2004;26:387–404. - PubMed
-
- Telci D, Griffin M. Tissue transglutaminase (TG2)–a wound response enzyme. Front Biosci. 2006;11:867–882. - PubMed
-
- Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, et al. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001;276:20673–20678. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

