Choreographing metastasis to the tune of LTBP
- PMID: 21494784
- PMCID: PMC3747963
- DOI: 10.1007/s10911-011-9215-3
Choreographing metastasis to the tune of LTBP
Abstract
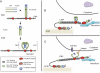
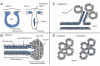
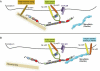
Latent Transforming Growth Factor beta (TGFβ) Binding Proteins (LTBPs) are chaperones and determinants of TGFβ isoform-specific secretion. They belong to the LTBP/Fibrillin family and form integral components of the fibronectin and microfibrillar extracellular matrix (ECM). LTBPs serve as master regulators of TGFβ bioavailability, functioning to incorporate and spatially pattern latent TGFβ at regular intervals within the ECM, and actively participate in integrin-mediated stretch activation of TGFβ in vivo. In so doing they create a highly patterned sensory system where local changes in ECM tension can be detected and transduced into focal signals. The physiological role of LTBPs in the mammary gland remains largely unstudied, however both loss and gain of LTBP expression is found in breast cancers and breast cancer cell lines. Importantly, elevated LTBP1 levels appear in two gene signatures predictive of enhanced metastatic behavior. LTBP may promote metastasis by providing the bridge between structural and signaling components of the epithelial to mesenchymal transition (EMT).
Figures

References
-
- Gorska AE, Joseph H, Derynck R, Moses HL, Serra R. Dominant-negative interference of the transforming growth factor beta type II receptor in mammary gland epithelium results in alveolar hyperplasia and differentiation in virgin mice. Cell Growth Differ. 1998;9(3):229–38. - PubMed
-
- Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41(3):233–64. - PubMed
-
- Robinson SD, Silberstein GB, Roberts AB, Flanders KC, Daniel CW. Regulated expression and growth inhibitory effects of transforming growth factor-beta isoforms in mouse mammary gland development. Development. 1991;113(3):867–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

