Carbene catalysts
- PMID: 21494949
- PMCID: PMC3820033
- DOI: 10.1007/978-3-642-02815-1_18
Carbene catalysts
Abstract
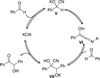
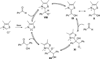
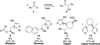
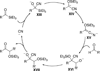
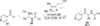
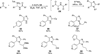
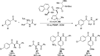
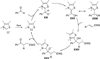
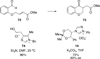
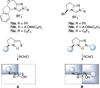
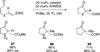
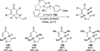
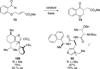
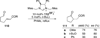
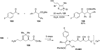
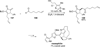
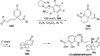
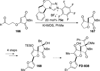
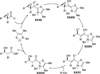
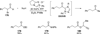
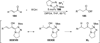
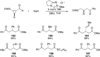
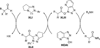
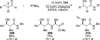
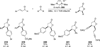
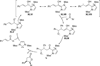
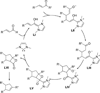
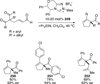
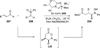
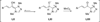
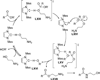
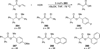
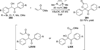
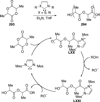
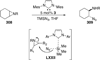
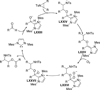
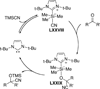
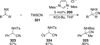
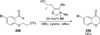
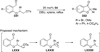
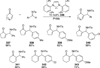
The use of N-heterocyclic carbenes as catalysts for organic transformations has received increased attention in the past 10 years. A discussion of catalyst development and nucleophilic characteristics precedes a description of recent advancements and new reactions using N-heterocyclic carbenes in catalysis.
Figures


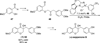

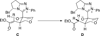


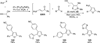
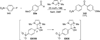
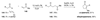
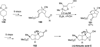



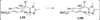

References
-
- Arduengo AJ, III, Harlow RL, Kline M. J Am Chem Soc. 1991;113:361.
-
- Ukai T, Tanaka R, Dokawa T. J Pharm Soc Jpn. 1943;63:296.
-
- Sheehan JC, Hunneman DH. J Am Chem Soc. 1966;88:3666.
-
- Christmann M. Angew Chem, Int Ed Engl. 2005;44:2632. - PubMed
-
- Enders D, Balensiefer T. Acc Chem Res. 2004;37:534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

