Total (bio)synthesis: strategies of nature and of chemists
- PMID: 21495259
- PMCID: PMC3109256
- DOI: 10.1007/128_2010_79
Total (bio)synthesis: strategies of nature and of chemists
Abstract
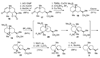
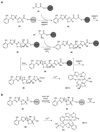
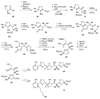
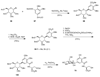
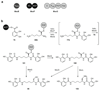
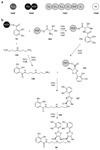
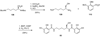
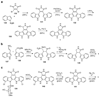
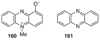
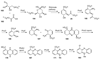
The biosynthetic pathways to a number of natural products have been reconstituted in vitro using purified enzymes. Many of these molecules have also been synthesized by organic chemists. Here we compare the strategies used by nature and by chemists to reveal the underlying logic and success of each total synthetic approach for some exemplary molecules with diverse biosynthetic origins.
Figures


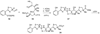


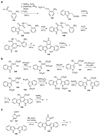

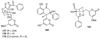
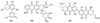
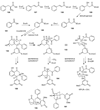
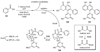


Similar articles
-
Stereocontrolled total synthesis of natural products with characteristic molecular structures and biological activities.Chem Pharm Bull (Tokyo). 2013;61(8):781-98. doi: 10.1248/cpb.c13-00417. Chem Pharm Bull (Tokyo). 2013. PMID: 23902860 Review.
-
Collective synthesis of natural products by means of organocascade catalysis.Nature. 2011 Jul 13;475(7355):183-8. doi: 10.1038/nature10232. Nature. 2011. PMID: 21753848 Free PMC article.
-
Biomimetic Assembly Lines Producing Natural Product Analogs: Strategies from a Versatile Manifold to Skeletally Diverse Scaffolds.Chem Rec. 2016 Apr;16(2):652-66. doi: 10.1002/tcr.201500213. Epub 2016 Feb 1. Chem Rec. 2016. PMID: 26828424
-
Biomimetic syntheses of the neurotrophic natural products caryolanemagnolol and clovanemagnolol.Org Lett. 2010 Mar 19;12(6):1304-7. doi: 10.1021/ol100214x. Org Lett. 2010. PMID: 20158263
-
Ruthenium-Catalyzed Metathesis Cascade Reactions in Natural Products Synthesis.Chem Rec. 2017 May;17(5):499-517. doi: 10.1002/tcr.201600110. Epub 2016 Oct 24. Chem Rec. 2017. PMID: 27775863 Review.
Cited by
-
Complexity reduction and opportunities in the design, integration and intensification of biocatalytic processes for metabolite synthesis.Front Bioeng Biotechnol. 2022 Jul 22;10:958606. doi: 10.3389/fbioe.2022.958606. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35935499 Free PMC article. Review.
-
A comprehensive review of the anticancer effects of decursin.Front Pharmacol. 2024 Feb 20;15:1303412. doi: 10.3389/fphar.2024.1303412. eCollection 2024. Front Pharmacol. 2024. PMID: 38444945 Free PMC article. Review.
-
Cell-Free Total Biosynthesis of Plant Terpene Natural Products using an Orthogonal Cofactor Regeneration System.ACS Catal. 2021 Aug 6;11(15):9898-9903. doi: 10.1021/acscatal.1c02267. Epub 2021 Jul 23. ACS Catal. 2021. PMID: 35355836 Free PMC article.
-
Charting the Evolution of Chemoenzymatic Strategies in the Syntheses of Complex Natural Products.J Am Chem Soc. 2023 Aug 23;145(33):18161-18181. doi: 10.1021/jacs.3c03422. Epub 2023 Aug 8. J Am Chem Soc. 2023. PMID: 37553092 Free PMC article. Review.
-
A Promiscuous Bacterial P450: The Unparalleled Diversity of BM3 in Pharmaceutical Metabolism.Int J Mol Sci. 2021 Oct 21;22(21):11380. doi: 10.3390/ijms222111380. Int J Mol Sci. 2021. PMID: 34768811 Free PMC article. Review.
References
-
- Nicolaou KC, Vourloumis D, Winssinger N, Baran PS. Angew Chem Int Ed. 2000;39:44–122. - PubMed
-
- Gaudêncio SP, Santos MMM, Lobo AM, Prabhakar S. Tetrahedron Lett. 2003;44:2577–2578.
-
- Mahboobi S, Eibler E, Koller M, Kumar S, Popp A, Schollmeyer D. J Org Chem. 1999;64:4697–4704. - PubMed
-
- Beccalli EM, Gelmi ML, Marchesini A. Tetrahedron. 1998;54:6909–6918.
-
- Wood JL, Stoltz BM, Dietrich HJ. J Am Chem Soc. 1995;117:10413–10414.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

