Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats
- PMID: 21495821
- PMCID: PMC3166387
- DOI: 10.3171/2011.3.JNS101721
Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats
Abstract
Object: Delayed (24 hours postinjury) treatment with erythropoietin (EPO) improves functional recovery following experimental traumatic brain injury (TBI). In this study, the authors tested whether therapeutic effects of delayed EPO treatment for TBI are dose dependent in an attempt to establish an optimal dose paradigm for the delayed EPO treatment.
Methods: Experimental TBI was performed in anesthetized young adult male Wistar rats using a controlled cortical impact device. Sham animals underwent the same surgical procedure without injury. The animals (8 rats/group) received 3 intraperitoneal injections of EPO (0, 1000, 3000, 5000, or 7000 U/kg body weight, at 24, 48, and 72 hours) after TBI. Sensorimotor and cognitive functions were assessed using a modified neurological severity score and foot fault test, and Morris water maze tests, respectively. Animals were killed 35 days after injury, and the brain sections were stained for immunohistochemical analyses.
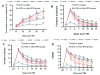
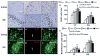
Results: Compared with the saline treatment, EPO treatment at doses from 1000 to 7000 U/kg did not alter lesion volume but significantly reduced hippocampal neuron loss, enhanced angiogenesis and neurogenesis in the injured cortex and hippocampus, and significantly improved sensorimotor function and spatial learning. The animals receiving the medium dose of 5000 U/kg exhibited a significant improvement in histological and functional outcomes compared with the lower or higher EPO dose groups.
Conclusions: These data demonstrate that delayed (24 hours postinjury) treatment with EPO provides dose-dependent neurorestoration, which may contribute to improved functional recovery after TBI, implying that application of an optimal dose of EPO is likely to increase successful preclinical and clinical trials for treatment of TBI.
Figures
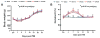

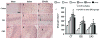

Similar articles
-
Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury.J Neurosurg. 2011 Feb;114(2):549-59. doi: 10.3171/2010.10.JNS10925. Epub 2010 Nov 12. J Neurosurg. 2011. PMID: 21073254 Free PMC article.
-
Impact of inhibition of erythropoietin treatment-mediated neurogenesis in the dentate gyrus of the hippocampus on restoration of spatial learning after traumatic brain injury.Exp Neurol. 2012 May;235(1):336-44. doi: 10.1016/j.expneurol.2012.02.015. Epub 2012 Mar 4. Exp Neurol. 2012. PMID: 22414310 Free PMC article.
-
Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit.Brain Res. 2009 Oct 19;1294:153-64. doi: 10.1016/j.brainres.2009.07.077. Epub 2009 Jul 30. Brain Res. 2009. PMID: 19646970 Free PMC article.
-
Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose.J Neurosurg. 2010 Sep;113(3):598-608. doi: 10.3171/2009.9.JNS09844. J Neurosurg. 2010. PMID: 19817538 Free PMC article.
-
Emerging treatments for traumatic brain injury.Expert Opin Emerg Drugs. 2009 Mar;14(1):67-84. doi: 10.1517/14728210902769601. Expert Opin Emerg Drugs. 2009. PMID: 19249984 Free PMC article. Review.
Cited by
-
Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain.Front Cell Neurosci. 2013 Jan 18;6:70. doi: 10.3389/fncel.2012.00070. eCollection 2012. Front Cell Neurosci. 2013. PMID: 23346046 Free PMC article.
-
Response of the cerebral vasculature following traumatic brain injury.J Cereb Blood Flow Metab. 2017 Jul;37(7):2320-2339. doi: 10.1177/0271678X17701460. Epub 2017 Apr 5. J Cereb Blood Flow Metab. 2017. PMID: 28378621 Free PMC article. Review.
-
Animal models of traumatic brain injury.Nat Rev Neurosci. 2013 Feb;14(2):128-42. doi: 10.1038/nrn3407. Nat Rev Neurosci. 2013. PMID: 23329160 Free PMC article. Review.
-
Comparison of the effects of erythropoietin and anakinra on functional recovery and gene expression in a traumatic brain injury model.Front Pharmacol. 2013 Oct 17;4:129. doi: 10.3389/fphar.2013.00129. eCollection 2013. Front Pharmacol. 2013. PMID: 24151467 Free PMC article.
-
EPO improved neurologic outcome in rat pups late after traumatic brain injury.Brain Dev. 2018 May;40(5):367-375. doi: 10.1016/j.braindev.2018.01.003. Epub 2018 Feb 21. Brain Dev. 2018. PMID: 29429559 Free PMC article.
References
-
- Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav Brain Res. 1990;39:73–95. - PubMed
-
- Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. - PubMed
-
- Cariou A, Claessens YE, Pene F, Marx JS, Spaulding C, Hababou C, et al. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: a matched control study. Resuscitation. 2008;76:397–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

