Reporting from the field: genetically encoded fluorescent reporters uncover signaling dynamics in living biological systems
- PMID: 21495849
- PMCID: PMC4384825
- DOI: 10.1146/annurev-biochem-060409-093259
Reporting from the field: genetically encoded fluorescent reporters uncover signaling dynamics in living biological systems
Abstract
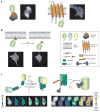
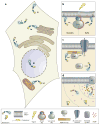
Real-time visualization of a wide range of biochemical processes in living systems is being made possible through the development and application of genetically encoded fluorescent reporters. These versatile biosensors have proven themselves tailor-made to the study of signal transduction, and in this review, we discuss some of the unique insights that they continue to provide regarding the spatial organization and dynamic regulation of intracellular signaling networks. In addition, we explore the more recent push to expand the scope of biological phenomena that can be monitored using these reporters, while also considering the potential to integrate this highly adaptable technology with a number of emerging techniques that may significantly broaden our view of how networks of biochemical processes shape larger biological phenomena.
Figures

References
-
- Coons AH, Creech HJ, Jones RN, Berliner E. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159–70.
-
- Bloom FE, Wedner HJ, Parker CW. The use of antibodies to study cell structure and metabolism. Pharmacol Rev. 1973;25:343–58. - PubMed
-
- Heffetz D, Fridkin M, Zick Y. Antibodies directed against phosphothreonine residues as potent tools for studying protein phosphorylation. Eur J Biochem. 1989;182:343–48. - PubMed
-
- Kaufmann H, Bailey JE, Fussenegger M. Use of antibodies for detection of phosphorylated proteins separated by two-dimensional gel electrophoresis. Proteomics. 2001;1:194–99. - PubMed
-
- Ross AH, Baltimore D, Eisen HN. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981;294:654–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

