The transfusion problem: role of aberrant S-nitrosylation
- PMID: 21496046
- PMCID: PMC3589136
- DOI: 10.1111/j.1537-2995.2011.03097.x
The transfusion problem: role of aberrant S-nitrosylation
Abstract
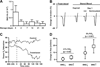
Protein S-nitrosylation (the binding of a nitric oxide [NO] group to a cysteine thiol) is a major mechanism through which the ubiquitous cellular influence of NO is exerted. Disruption of S-nitrosylation is associated with a wide range of pathophysiologic conditions. Hemoglobin (Hb) exemplifies both of these concepts. It is the prototypical S-nitrosylated protein in that it binds, activates, and deploys NO. Within red blood cells (RBCs), Hb is S-nitrosylated during the respiratory cycle and thereby conveys NO bioactivity that may be dispensed to regulate local blood flow in the physiologic response known as hypoxic vasodilation. Hb thus both delivers oxygen directly and delivers vasoactivity to potentially optimize tissue perfusion in concert with local metabolic demand. Accordingly, decreased levels of S-nitrosylated Hb (also known as S-nitrosohemoglobin) and/or impaired delivery of RBC-derived NO bioactivity have been observed in a variety of disease states that are characterized by tissue hypoxemia. It has been shown recently that storage of blood depletes S-nitrosylated Hb, accompanied by reduced ability of RBCs to induce vasodilation. This defect appears to account in significant part for the impaired ability of banked RBCs to deliver oxygen. Renitrosylation can correct this impairment and thus may offer a means to ameliorate the disruptions in tissue perfusion produced by transfusion.
© 2011 American Association of Blood Banks.
Figures
References
-
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–526. - PubMed
-
- McMahon TJ, Exton Stone A, Bonaventura J, Singel DJ, Solomon Stamler J. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J Biol Chem. 2000;275(22):16738–16745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

