Effects of strong CYP2D6 and 3A4 inhibitors, paroxetine and ketoconazole, on the pharmacokinetics and cardiovascular safety of tamsulosin
- PMID: 21496064
- PMCID: PMC3162654
- DOI: 10.1111/j.1365-2125.2011.03988.x
Effects of strong CYP2D6 and 3A4 inhibitors, paroxetine and ketoconazole, on the pharmacokinetics and cardiovascular safety of tamsulosin
Abstract
What is already known about this subject: Tamsulosin metabolism involves both CYP2D6 and 3A4. However, data on potential drug-drug interactions between tamsulosin and inhibitors of CYP2D6 and 3A4 are limited and information on potential pharmacodynamic consequences of such pharmacokinetic interactions is missing.
What this study adds: This study provides information on the drug-drug interactions of tamsulosin with strong CYP2D6 and strong CYP3A4 inhibitors after single dose administration in healthy subjects.
Aim: To determine the effect of the strong CYP2D6 inhibitor paroxetine and strong CYP3A4 inhibitor ketoconazole on the pharmacokinetics and safety (orthostatic challenge) of tamsulosin.
Methods: Two open-label, randomized, two-way crossover studies were conducted in healthy male volunteers (extensive CYP2D6 metabolizers).
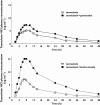
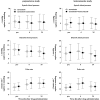
Results: Co-administration of multiple oral doses of 20 mg paroxetine once daily with a single oral dose of the 0.4 mg tamsulosin HCl capsule increased the adjusted geometric mean (gMean) values of C(max) and AUC(0,∞) of tamsulosin by factors of 1.34 (90% CI 1.21, 1.49) and 1.64 (90% CI 1.44, 1.85), respectively, and increased the terminal half-life (t(1/2) ) of tamsulosin HCl from 11.4 h to 15.3 h. Co-administration of multiple oral doses of 400 mg ketoconazole once daily with a single oral dose of the 0.4 mg tamsulosin increased the gMean values of C(max) and AUC(0,∞) of tamsulosin by a factor of 2.20 (90% CI 1.96, 2.45) and 2.80 (90% CI 2.56, 3.07), respectively. The terminal half-life was slightly increased from 10.5 h to 11.8 h. These pharmacokinetic changes were not accompanied by clinically significant alterations of haemodynamic responses during orthostatic stress testing.
Conclusion: The exposure to tamsulosin is increased upon co-administration of strong CYP2D6 inhibitors and even more so of strong 3A4 inhibitors, but neither PK alteration was accompanied by clinically significant haemodynamic changes during orthostatic stress testing.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
Similar articles
-
Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone.Eur J Clin Pharmacol. 2011 Jan;67(1):63-71. doi: 10.1007/s00228-010-0893-3. Epub 2010 Sep 21. Eur J Clin Pharmacol. 2011. PMID: 20857093 Clinical Trial.
-
Role of cytochrome p450 isoenzymes 3A and 2D6 in the in vivo metabolism of mirabegron, a β3-adrenoceptor agonist.Clin Drug Investig. 2013 Jun;33(6):429-40. doi: 10.1007/s40261-013-0084-y. Clin Drug Investig. 2013. PMID: 23625188 Clinical Trial.
-
Effect of cytochrome P450 3A4 inhibitor ketoconazole on risperidone pharmacokinetics in healthy volunteers.J Clin Pharm Ther. 2012 Apr;37(2):221-5. doi: 10.1111/j.1365-2710.2011.01271.x. Epub 2011 Apr 26. J Clin Pharm Ther. 2012. PMID: 21518375 Clinical Trial.
-
Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone.Br J Clin Pharmacol. 2010 Jul;70(1):78-87. doi: 10.1111/j.1365-2125.2010.03653.x. Br J Clin Pharmacol. 2010. PMID: 20642550 Free PMC article. Clinical Trial.
-
The effect of ketoconazole on the pharmacokinetics and pharmacodynamics of inhaled fluticasone furoate and vilanterol trifenatate in healthy subjects.Br J Clin Pharmacol. 2013 Jun;75(6):1478-87. doi: 10.1111/bcp.12019. Br J Clin Pharmacol. 2013. PMID: 23116485 Free PMC article. Clinical Trial.
Cited by
-
Differential effects of the enantiomers of tamsulosin and tolterodine on P-glycoprotein and cytochrome P450 3A4.Naunyn Schmiedebergs Arch Pharmacol. 2017 Jan;390(1):49-59. doi: 10.1007/s00210-016-1304-9. Epub 2016 Sep 27. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 27678410
-
Clinical pharmacology of functional disorders of the urogenital system.Br J Clin Pharmacol. 2011 Aug;72(2):183-5. doi: 10.1111/j.1365-2125.2011.04013.x. Br J Clin Pharmacol. 2011. PMID: 21564172 Free PMC article. No abstract available.
-
Physiologically based pharmacokinetic (PBPK) modelling of tamsulosin related to CYP2D6*10 allele.Arch Pharm Res. 2021 Nov;44(11):1037-1049. doi: 10.1007/s12272-021-01357-z. Epub 2021 Nov 9. Arch Pharm Res. 2021. PMID: 34751931 Clinical Trial.
-
Stability-Indicating RP-HPLC Method for the Simultaneous Determination of Prazosin, Terazosin, and Doxazosin in Pharmaceutical Formulations.Sci Pharm. 2012 Jul-Sep;80(3):619-31. doi: 10.3797/scipharm.1204-15. Epub 2012 May 22. Sci Pharm. 2012. PMID: 23008810 Free PMC article.
-
Population pharmacokinetics of tamsulosine in patients with benign prostatic hyperplasia.World J Urol. 2024 Jul 22;42(1):427. doi: 10.1007/s00345-024-05115-w. World J Urol. 2024. PMID: 39037497
References
-
- Kok ET, Schouten BW, Bohnen AM, Groeneveld FPMW, Thomas S, Bosch JLHR. Risk factors for lower urinary tract symptoms suggestive of benign prostatic hyperplasia in a community based population of healthy aging men: the Krimpen study. J Urol. 2009;181:710–6. - PubMed
-
- Sarma AV, Jacobson DJ, McGree ME, Roberts RO, Lieber MM, Jacobsen SJ. A population based study of incidence and treatment of benign prostatic hyperplasia among residents of Olmsted County, Minnesota: 1987 to 1997. J Urol. 2005;173:2048–53. - PubMed
-
- Naslund MJ, Miner M. A review of the clinical efficacy and safety of 5α-reductase inhibitors for the enlarged prostate. Clin Ther. 2007;29:17–25. - PubMed
-
- Michel MC. The forefront of novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: α-blockers in the treatment of male voiding dysfunction – How do they work and why do they differ in tolerability? J Pharmacol Sci. 2010;112:151–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

