Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection
- PMID: 21496117
- PMCID: PMC3258267
- DOI: 10.1111/j.1399-3089.2011.00633.x
Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection
Erratum in
- Xenotransplantation. 2011 Jul-Aug;18(4):267
Abstract
Background: Galactosyl transferase gene knock-out (GalTKO) swine offer a unique tool to evaluate the role of the Gal antigen in xenogenic lung hyperacute rejection.
Methods: We perfused GalTKO miniature swine lungs with human blood. Results were compared with those from previous studies using wild-type and human decay-accelerating factor-transgenic (hDAF(+/+) ) pig lungs.
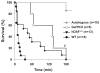
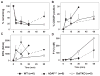
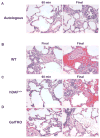
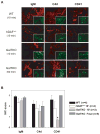
Results: GalTKO lungs survived 132 ± 52 min compared to 10 ± 9 min for wild-type lungs (P = 0.001) and 45 ± 60 min for hDAF(+/+) lungs (P = 0.18). GalTKO lungs displayed stable physiologic flow and pulmonary vascular resistance (PVR) until shortly before graft demise, similar to autologous perfusion, and unlike wild-type or hDAF(+/+) lungs. Early (15 and 60 min) complement (C3a) and platelet activation and intrapulmonary platelet deposition were significantly diminished in GalTKO lungs relative to wild-type or hDAF(+/+) lungs. However, GalTKO lungs adsorbed cytotoxic anti-non-Gal antibody and elaborated high levels of thrombin; their demise was associated with increased PVR, capillary congestion, intravascular thrombi and strong CD41 deposition not seen at earlier time points.
Conclusions: In summary, GalTKO lungs are substantially protected from injury but, in addition to anti-non-Gal antibody and complement, platelet adhesion and non-physiologic intravascular coagulation contribute to Gal-independent lung injury mechanisms.
© 2011 John Wiley & Sons A/S.
Figures




References
-
- Cooper DKC. Xenografting: how great is the clinical need? Xeno. 1993;1:25–26.
-
- Cooper DKC, Ye Y, Rolf JRLL, Zuhdi YYEN. The pig as potential organ donor for man. In: Cooper DKC, Kemp E, Reemtsma K, White DJG, editors. Xeno-Transplantation. Berlin: Springer-Verlag; 1991. pp. 481–500.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

