Exercise and nutrition routine improving cancer health (ENRICH): the protocol for a randomized efficacy trial of a nutrition and physical activity program for adult cancer survivors and carers
- PMID: 21496251
- PMCID: PMC3101179
- DOI: 10.1186/1471-2458-11-236
Exercise and nutrition routine improving cancer health (ENRICH): the protocol for a randomized efficacy trial of a nutrition and physical activity program for adult cancer survivors and carers
Abstract
Background: The Exercise and Nutrition Routine Improving Cancer Health (ENRICH) study is investigating a novel lifestyle intervention aimed at improving the health behaviors of adult cancer survivors and their carers. The main purpose of the study is to determine the efficacy of lifestyle education and skill development delivered via group-based sessions on the physical activity and dietary behaviors of participants. This article describes the intervention development, study design, and participant recruitment.
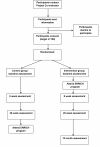
Methods/design: ENRICH is a randomized controlled trial, conducted in Australia, with two arms: an intervention group participating in six, two-hour face-to-face sessions held over eight weeks, and a wait-list control group. Intervention sessions are co-facilitated by an exercise physiologist and dietician. Content includes healthy eating education, and a home-based walking (utilizing a pedometer) and resistance training program (utilizing elastic tubing resistance devices). The program was developed with reference to social cognitive theory and chronic disease self-management models. The study population consists of cancer survivors (post active-treatment) and their carers recruited through community-based advertising and referral from health professionals. The primary outcome is seven-days of sealed pedometry. Secondary outcomes include: self-reported physical activity levels, dietary intake, sedentary behavior, waist circumference, body mass index, quality of life, and perceived social support. The outcomes will be measured at baseline (one week prior to attending the program), eight-weeks (at completion of intervention sessions), and 20-weeks. The intervention group will also be invited to complete 12-month follow-up data collection. Process evaluation data will be obtained from participants by questionnaire and attendance records.
Discussion: No trials are yet available that have evaluated the efficacy of group-based lifestyle education and skill development amongst mixed groups of cancer survivors and their carers. The results will have implications for the planning and provision of health and support services during the cancer survivorship phase.
Clinical trials registration: Australian New Zealand Clinical Trials Register identifier: ANZCTRN12609001086257.
Similar articles
-
Impact of a nutrition and physical activity intervention (ENRICH: Exercise and Nutrition Routine Improving Cancer Health) on health behaviors of cancer survivors and carers: a pragmatic randomized controlled trial.BMC Cancer. 2015 Oct 15;15:710. doi: 10.1186/s12885-015-1775-y. BMC Cancer. 2015. PMID: 26471791 Free PMC article. Clinical Trial.
-
Social cognitive theory mediators of physical activity in a lifestyle program for cancer survivors and carers: findings from the ENRICH randomized controlled trial.Int J Behav Nutr Phys Act. 2016 Apr 14;13:49. doi: 10.1186/s12966-016-0372-z. Int J Behav Nutr Phys Act. 2016. PMID: 27075417 Free PMC article. Clinical Trial.
-
Maintenance of Lifestyle Changes at 12-month Follow-up in a Nutrition and Physical Activity Trial for Cancer Survivors.Am J Health Behav. 2017 Nov 1;41(6):784-795. doi: 10.5993/AJHB.41.6.12. Am J Health Behav. 2017. PMID: 29025506 Clinical Trial.
-
Using the TIDieR checklist to describe development and integration of a web-based intervention promoting healthy eating and regular exercise among older cancer survivors.Digit Health. 2023 Jun 26;9:20552076231182805. doi: 10.1177/20552076231182805. eCollection 2023 Jan-Dec. Digit Health. 2023. PMID: 37434730 Free PMC article. Review.
-
Weight management and physical activity throughout the cancer care continuum.CA Cancer J Clin. 2018 Jan;68(1):64-89. doi: 10.3322/caac.21441. Epub 2017 Nov 22. CA Cancer J Clin. 2018. PMID: 29165798 Free PMC article. Review.
Cited by
-
A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors.J Cancer Surviv. 2015 Jun;9(2):305-38. doi: 10.1007/s11764-014-0413-z. Epub 2014 Nov 29. J Cancer Surviv. 2015. PMID: 25432633 Free PMC article.
-
Challenges in Cancer Self-management of Patients with Limited English Proficiency.Asia Pac J Oncol Nurs. 2016 Jul-Sep;3(3):259-265. doi: 10.4103/2347-5625.189815. Asia Pac J Oncol Nurs. 2016. PMID: 27981169 Free PMC article.
-
Wearable activity monitors in oncology trials: Current use of an emerging technology.Contemp Clin Trials. 2018 Jan;64:13-21. doi: 10.1016/j.cct.2017.11.002. Epub 2017 Nov 9. Contemp Clin Trials. 2018. PMID: 29129704 Free PMC article. Review.
-
Cancer caregivers' perceptions of an exercise and nutrition program.Support Care Cancer. 2013 Mar;21(3):803-10. doi: 10.1007/s00520-012-1583-8. Epub 2012 Sep 7. Support Care Cancer. 2013. PMID: 22956192
-
Demographic, clinical, psychosocial, and environmental correlates of objectively assessed physical activity among breast cancer survivors.Support Care Cancer. 2016 Aug;24(8):3333-42. doi: 10.1007/s00520-016-3148-8. Epub 2016 Mar 12. Support Care Cancer. 2016. PMID: 26970957 Free PMC article.
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide. IARC CancerBase No 10. Lyon: IARC; 2010.
-
- AIHW (Australian Institute of Health and Welfare), AACR (Australasian Association of Cancer Registries) Cancer in Australia: an overview, 2006. Canberra: AIHW; 2007.
-
- Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor. Lost in transition. Washington DC: The National Academies Press; 2005.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical