High-dose tranexamic acid reduces blood loss in postpartum haemorrhage
- PMID: 21496253
- PMCID: PMC3219400
- DOI: 10.1186/cc10143
High-dose tranexamic acid reduces blood loss in postpartum haemorrhage
Abstract
Introduction: Our purpose in conducting this study was to determine whether administration of high-dose tranexamic acid (TA) at the time of diagnosis of postpartum haemorrhage (PPH) could reduce blood loss.
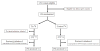
Methods: This was a randomised, controlled, multicentred, open-label trial. Women with PPH >800 mL following vaginal delivery were randomly assigned to receive TA (loading dose 4 g over 1 hour, then infusion of 1 g/hour over 6 hours) or not. In both groups, packed red blood cells (PRBCs) and colloids could be used according to French guidelines. The use of additional procoagulant treatments was permitted only in cases involving intractable bleeding. The primary objective was to assess the efficacy of TA in the reduction of blood loss in women with PPH, and the secondary objectives were the effect of TA on PPH duration, anaemia, transfusion and the need for invasive procedures.
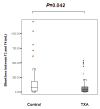
Results: A total of 144 women fully completed the protocol (72 in each group). Blood loss between enrolment and 6 hours later was significantly lower in the TA group than in the control group (median, 173 mL; first to third quartiles, 59 to 377) than in controls (221 mL; first to third quartiles 105 to 564) (P = 0.041). In the TA group, bleeding duration was shorter and progression to severe PPH and PRBC transfusion was less frequent than in controls (P < 0.03). Invasive procedures were performed in four women in the TA group and in seven controls (P = NS). PPH stopped after only uterotonics and PRBC transfusion in 93% of women in the TA group versus 79% of controls (P = 0.016). Mild, transient adverse manifestations occurred more often in the TA group than in the control group (P = 0.03).
Conclusions: This study is the first to demonstrate that high-dose TA can reduce blood loss and maternal morbidity in women with PPH. Although the study was not adequately powered to address safety issues, the observed side effects were mild and transient. A larger international study is needed to investigate whether TA can decrease the need for invasive procedures and reduce maternal morbidity in women with PPH.
Trial registration: Controlled Trials ISRCTN09968140.
Figures



References
-
- Dupont C, Touzet S, Colin C, Deneux-Tharaux C, Rabilloud M, Clement HJ, Lansac J, Colle MH, Rudigoz RC. Groupe PITHAGORE 6. Incidence and management of postpartum haemorrhage following the dissemination of guidelines in a network of 16 maternity units in France. Int J Obstet Anesth. 2009;18:320–327. doi: 10.1016/j.ijoa.2009.02.017. - DOI - PubMed
-
- Lewis G, (Ed) The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers Lives: Reviewing Maternal Deaths to Make Childhood Safer: 2003-2005. London: CEMACH; 2007. http://www.cemach.org.uk/getattachment/26dae364-1fc9-4a29-a6cb-afb3f251f...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

