Tenascin-C and alpha-smooth muscle actin positive cells are increased in the large airways in patients with COPD
- PMID: 21496259
- PMCID: PMC3083344
- DOI: 10.1186/1465-9921-12-48
Tenascin-C and alpha-smooth muscle actin positive cells are increased in the large airways in patients with COPD
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is characterized by inflammation and remodeling of the lungs. This results in alterations in extracellular matrix (ECM) and structural changes leading to airflow obstruction. We studied the expression of tenascin-C (Tn-C) and alpha smooth muscle actin (α-SMA), which act as a marker of myofibroblasts, in large airways from COPD patients. Our aim was to elucidate whether this expression correlated with smoking or with disease development.
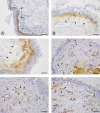
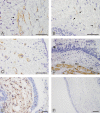
Methods: Bronchoscopy was performed on 20 COPD patients (mean age 56 years; range 39-61; FEV1/FVC < 70% and FEV1 median 53% (range 33-69) of predicted). Age and smoking matched smokers (S) without COPD (n = 13) and age matched non-smokers (NS) (n = 14) served as controls. Bronchial mucosal biopsies were analyzed by immunohistochemistry. The distribution of Tn-C expression was assessed and graded in three levels, and the number of spindle shaped cells staining positive for α-SMA were counted.
Results: Biopsies from COPD patients had more (P < 0.001) Tn-C expression than the two control groups. A significantly (P < 0.05) increased number of spindle shaped cells expressing α-SMA was observed in COPD patients compared with the controls. Smokers and nonsmokers did not differ in this respect. The expression of Tn-C correlated positively (P < 0.001) to the number of α-SMA positive cells.
Conclusions: We demonstrate increased expression of Tn-C and α-SMA positive cells in the large airways in COPD. This was not associated to smoking per se, but to the presence of airway obstruction. Our findings add new information regarding remodeling characteristics and highlight the large airways as a potential site for airways obstruction in COPD.
Figures

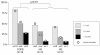

Similar articles
-
Central airways remodeling in COPD patients.Int J Chron Obstruct Pulmon Dis. 2014 Sep 1;9:927-32. doi: 10.2147/COPD.S52478. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 25214779 Free PMC article.
-
ADAM15 expression is increased in lung CD8+ T cells, macrophages, and bronchial epithelial cells in patients with COPD and is inversely related to airflow obstruction.Respir Res. 2020 Jul 16;21(1):188. doi: 10.1186/s12931-020-01446-5. Respir Res. 2020. PMID: 32677970 Free PMC article.
-
Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD).Am J Respir Crit Care Med. 2001 May;163(6):1476-83. doi: 10.1164/ajrccm.163.6.9908135. Am J Respir Crit Care Med. 2001. PMID: 11371421
-
Myofibroblast expression in airways and alveoli is affected by smoking and COPD.Respir Res. 2013 Aug 11;14(1):84. doi: 10.1186/1465-9921-14-84. Respir Res. 2013. PMID: 23937155 Free PMC article.
-
Increased human Ca²⁺-activated Cl⁻ channel 1 expression and mucus overproduction in airway epithelia of smokers and chronic obstructive pulmonary disease patients.Respir Res. 2012 Jun 25;13(1):55. doi: 10.1186/1465-9921-13-55. Respir Res. 2012. PMID: 22731784 Free PMC article.
Cited by
-
Mechanical Compression of Human Airway Epithelial Cells Induces Release of Extracellular Vesicles Containing Tenascin C.Cells. 2022 Jan 13;11(2):256. doi: 10.3390/cells11020256. Cells. 2022. PMID: 35053372 Free PMC article.
-
Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease.Cells. 2021 Jun 26;10(7):1602. doi: 10.3390/cells10071602. Cells. 2021. PMID: 34206722 Free PMC article. Review.
-
Tenascin-C serum levels and its prognostic power in non-small cell lung cancer.Oncotarget. 2016 Apr 12;7(15):20945-52. doi: 10.18632/oncotarget.7976. Oncotarget. 2016. PMID: 26967391 Free PMC article.
-
Identification of factors directly linked to incident chronic obstructive pulmonary disease: A causal graph modeling study.PLoS Med. 2024 Aug 13;21(8):e1004444. doi: 10.1371/journal.pmed.1004444. eCollection 2024 Aug. PLoS Med. 2024. PMID: 39137208 Free PMC article.
-
Tenascin-C: Friend or Foe in Lung Aging?Front Physiol. 2021 Oct 27;12:749776. doi: 10.3389/fphys.2021.749776. eCollection 2021. Front Physiol. 2021. PMID: 34777012 Free PMC article.
References
-
- Lindberg A, Bjerg A, Ronmark E, Larsson LG, Lundback B. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2006;100:264–272. doi: 10.1016/j.rmed.2005.04.029. - DOI - PubMed
-
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. - DOI - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.com/GuidelinesResources.asp - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

