Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emissions
- PMID: 21496448
- PMCID: PMC3083828
Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emissions
Abstract
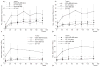
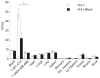
Imaging the location and extent of cancer provides invaluable information before, during, and after surgery. The majority of "image-guided" methods that use, for example, positron emission tomography (PET) involve preoperative imaging and do not provide real-time information during surgery. It is now well established that the inherent optical emissions (Cerenkov radiation) from various β-emitting radionuclides can be visualized by Cerenkov luminescence imaging (CLI). Here we report the full characterization of CLI using the positron-emitting radiotracer 89Zr-DFO-trastuzumab for target-specific, quantitative imaging of HER2/neu-positive tumors in vivo. We also provide the first demonstration of the feasibility of using CLI for true image-guided, intraoperative surgical resection of tumors. Analysis of optical CLIs provided accurate, quantitative information on radiotracer biodistribution and tissue uptake that correlated well with the concordant PET images. CLI, PET, and biodistribution studies revealed target-specific uptake of 89Zr-DFO-trastuzumab in BT-474 (HER2/neu positive) versus MDA-MB-468 (HER2/neu negative) xenografts in the same mice. Competitive inhibition (blocking) studies followed by CLI also confirmed the in vivo immunoreactivity and specificity of 89Zr-DFO-trastuzumab for HER2/neu. Overall, these results strongly support the continued development of CLI as a preclinical and possible clinical tool for use in molecular imaging and surgical procedures for accurately defining tumor margins.
Figures

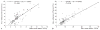

Similar articles
-
Cerenkov luminescence imaging of medical isotopes.J Nucl Med. 2010 Jul;51(7):1123-30. doi: 10.2967/jnumed.110.076521. Epub 2010 Jun 16. J Nucl Med. 2010. PMID: 20554722 Free PMC article.
-
Positron-Emission Tomography of HER2-Positive Breast Cancer Xenografts in Mice with 89Zr-Labeled Trastuzumab-DM1: A Comparison with 89Zr-Labeled Trastuzumab.Mol Pharm. 2018 Aug 6;15(8):3383-3393. doi: 10.1021/acs.molpharmaceut.8b00392. Epub 2018 Jul 16. Mol Pharm. 2018. PMID: 29957952
-
Cerenkov Luminescence Imaging as a Modality to Evaluate Antibody-Based PET Radiotracers.J Nucl Med. 2017 Jan;58(1):175-180. doi: 10.2967/jnumed.116.178780. Epub 2016 Aug 18. J Nucl Med. 2017. PMID: 27539844 Free PMC article.
-
Cerenkov luminescence imaging (CLI) for image-guided cancer surgery.Clin Transl Imaging. 2016;4(5):353-366. doi: 10.1007/s40336-016-0183-x. Epub 2016 May 24. Clin Transl Imaging. 2016. PMID: 27738626 Free PMC article. Review.
-
Cerenkov imaging.Adv Cancer Res. 2014;124:213-34. doi: 10.1016/B978-0-12-411638-2.00006-9. Adv Cancer Res. 2014. PMID: 25287690 Free PMC article. Review.
Cited by
-
Cerenkov Luminescence Imaging (CLI) for cancer therapy monitoring.J Vis Exp. 2012 Nov 13;(69):e4341. doi: 10.3791/4341. J Vis Exp. 2012. PMID: 23183774 Free PMC article.
-
Advancing theranostics with tumor-targeting peptides for precision otolaryngology.World J Otorhinolaryngol Head Neck Surg. 2016 Jul 26;2(2):98-108. doi: 10.1016/j.wjorl.2016.05.006. eCollection 2016 Jun. World J Otorhinolaryngol Head Neck Surg. 2016. PMID: 29204554 Free PMC article. Review.
-
Contemporary use of sentinel lymph node biopsy in the head and neck.World J Otorhinolaryngol Head Neck Surg. 2016 Jul 25;2(2):117-125. doi: 10.1016/j.wjorl.2016.05.008. eCollection 2016 Jun. World J Otorhinolaryngol Head Neck Surg. 2016. PMID: 29204556 Free PMC article.
-
Current clinical applications of Cerenkov luminescence for intraoperative molecular imaging.Eur J Nucl Med Mol Imaging. 2024 Aug;51(10):2931-2940. doi: 10.1007/s00259-024-06602-3. Epub 2024 Jan 20. Eur J Nucl Med Mol Imaging. 2024. PMID: 38243119 Free PMC article. Review.
-
Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery.Eur J Nucl Med Mol Imaging. 2013 Oct;40(11):1718-29. doi: 10.1007/s00259-013-2471-2. Epub 2013 Jun 19. Eur J Nucl Med Mol Imaging. 2013. PMID: 23778558
References
-
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. - PubMed
-
- Balch GC, Mithani SK, Simpson JK, Kelley MC. Accuracy of intraoperative gross examination of surgical margin status in women undergoing partial mastectomy for breast malignancy. Am Surg. 2005;71:22–7. - PubMed
-
- Chagpar A, Yen T, Sahin A, et al. Intraoperative margin assessment reduces reexcision rates in patients with ductal carcinoma in situ treated with breast-conserving surgery. Am J Surg. 2003;186:371–7. - PubMed
-
- Vermeeren L, Meinhardt W, Bex A, et al. Paraaortic sentinel lymph nodes: toward optimal detection and intraoperative localization using SPECT/CT and intraoperative real-time imaging. J Nucl Med. 2010;51:376–82. - PubMed
-
- Vermeeren L, Valdes Olmos RA, Klop WMC, et al. A portable g-camera for intraoperative detection of sentinel nodes in the head and neck region. J Nucl Med. 2010;51:700–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
