ATP-binding cassette transporters modulate both coelenterazine- and D-luciferin-based bioluminescence imaging
- PMID: 21496450
- PMCID: PMC4052835
ATP-binding cassette transporters modulate both coelenterazine- and D-luciferin-based bioluminescence imaging
Abstract
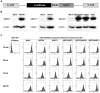
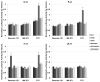
Bioluminescence imaging (BLI) of luciferase reporters provides a cost-effective and sensitive means to image biological processes. However, transport of luciferase substrates across the cell membrane does affect BLI readout intensity from intact living cells. To investigate the effect of ATP-binding cassette (ABC) transporters on BLI readout, we generated click beetle (cLuc), firefly (fLuc), Renilla (rLuc), and Gaussia (gLuc) luciferase HEK-293 reporter cells that overexpressed different ABC transporters (ABCB1, ABCC1, and ABCG2). In vitro studies showed a significant BLI intensity decrease in intact cells compared to cell lysates, when ABCG2 was overexpressed in HEK-293/cLuc, fLuc, and rLuc cells. Selective ABC transporter inhibitors were also applied. Inhibition of ABCG2 activity increased the BLI intensity more than two-fold in HEK-293/cLuc, fLuc, and rLuc cells; inhibition of ABCB1 elevated the BLI intensity two-fold only in HEK-293/rLuc cells. BLI of xenografts derived from HEK-293/ABC transporter/luciferase reporter cells confirmed the results of inhibitor treatment in vivo. These findings demonstrate that coelenterazine-based rLuc-BLI intensity can be modulated by ABCB1 and ABCG2. ABCG2 modulates d-luciferin-based BLI in a luciferase type-independent manner. Little ABC transporter effect on gLuc-BLI intensity is observed because a large fraction of gLuc is secreted. The expression level of ABC transporters is one key factor affecting BLI intensity, and this may be particularly important in luciferase-based applications in stem cell research.
Figures
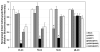


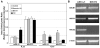
References
-
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. - PubMed
-
- Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–66. - PubMed
-
- Childs S, Ling V. The MDR superfamily of genes and its biological implications. Important Adv Oncol. 1994:21–36. - PubMed
-
- Dean M, Allikmets R. Evolution of ATP-binding cassette transporter genes. Curr Opin Genet Dev. 1995;5:779–85. - PubMed
-
- Fromm MF. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci. 2004;25:423–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
