Impaired odontogenic differentiation of senescent dental mesenchymal stem cells is associated with loss of Bmi-1 expression
- PMID: 21496667
- PMCID: PMC3079884
- DOI: 10.1016/j.joen.2011.02.009
Impaired odontogenic differentiation of senescent dental mesenchymal stem cells is associated with loss of Bmi-1 expression
Abstract
Introduction: Dental mesenchymal stem cells (dMSCs) might differentiate into odontoblast-like cells and form mineralized nodules. In the current study, we investigated the effects of senescence on odontogenic differentiation of dMSCs.
Methods: dMSCs were serially subcultured until senescence. Telomere lengths and telomerase activities were determined by quantitative polymerase chain reaction. Expression of genes involved in cell proliferation and differentiation, eg, Bmi-1, p16(INK4A), osteocalcin (OC), dentin sialoprotein (DSP), bone sialoprotein (BSP), and dentin matrix protein-1 (DMP-1) were assayed by Western blotting and quantitative reverse transcription polymerase chain reaction. Exogenous Bmi-1 was expressed in dMSCs by using retroviral vectors. Odontogenic differentiation was assayed by alkaline phosphatase activity.
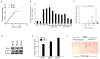
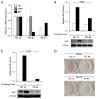
Results: Subculture-induced replicative senescence of dMSCs led to reduced expression of Bmi-1, OC, DSP, and BSP compared with rapidly proliferating cells, whereas p16(INK4A) level increased. The cells exhibited progressive loss of telomeric DNA during subculture, presumably as a result of lack of telomerase activity. Bmi-1 transduction did not affect proliferation of cells but enhanced the expression of OC and DSP in the late passage cultures. Bmi-1-transduced cells also demonstrated enhanced alkaline phosphatase activity and mineralized nodule formation.
Conclusions: These results indicate that dMSCs lose their odontogenic differentiation potential during senescence, in part by reduced Bmi-1 expression.
Copyright © 2011 American Association of Endodontists. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors deny any conflict of interest.
Figures

References
-
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. - PubMed
-
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

