Role of Klotho in aging, phosphate metabolism, and CKD
- PMID: 21496980
- PMCID: PMC3191324
- DOI: 10.1053/j.ajkd.2010.12.027
Role of Klotho in aging, phosphate metabolism, and CKD
Abstract
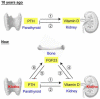
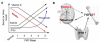
The klotho gene (KL) was identified first as a putative aging-suppressor gene that extended life span when overexpressed and accelerated aging-like phenotypes when disrupted in mice. It encodes a single-pass transmembrane protein and is expressed predominantly in kidney, where it functions as an obligate coreceptor for fibroblast growth factor 23 (FGF-23). FGF-23 is a bone-derived hormone that suppresses phosphate reabsorption and 1,25 dihydroxyvitamin D(3) (vitamin D) synthesis in the kidney. Klotho also is expressed in the parathyroid gland, where FGF-23 decreases parathyroid hormone expression and secretion, further suppressing vitamin D synthesis in kidney. Thus, FGF-23 functions as a phosphaturic hormone and a counter-regulatory hormone for vitamin D, thereby inducing negative phosphate balance. Mice lacking either FGF-23 or Klotho show hyperphosphatemia in addition to developing multiple aging-like phenotypes, which can be rescued by resolving phosphate retention. These findings have unveiled an unexpected link between aging and phosphate. In patients with chronic kidney disease (CKD), phosphate retention is seen universally and has been associated with increased mortality risk. Patients with CKD have high serum FGF-23 levels with decreased klotho expression in the kidney and parathyroid, rendering FGF-23 and Klotho as potential biomarkers and therapeutic targets for CKD. The Klotho protein not only serves as a coreceptor for FGF-23, but also functions as a humoral factor. Klotho's extracellular domain is released into blood and urine by ectodomain shedding and exerts various functions independently of FGF-23, including regulation of multiple ion channels and transporters. Decreased urinary Klotho protein level has been identified as one of the earliest biomarkers of CKD progression. This review focuses on the current understanding of Klotho protein function, with emphasis on its potential involvement in the pathophysiologic process of CKD.
Copyright © 2011 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures
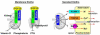
References
-
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. - PubMed
-
- Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–259. - PubMed
-
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627–2633. - PubMed
-
- Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–2138. - PubMed
-
- Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

