Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors
- PMID: 21497085
- PMCID: PMC3225230
- DOI: 10.1016/j.cub.2011.03.003
Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors
Abstract
Segregation of homologs at the first meiotic division (MI) is facilitated by crossovers and by a physical constraint imposed on sister kinetochores that facilitates monopolar attachment to the MI spindle. Recombination failure or premature separation of homologs results in univalent chromosomes at MI, and univalents constrained to form monopolar attachments should be inherently unstable and trigger the spindle assembly checkpoint (SAC). Although univalents trigger cell-cycle arrest in the male, this is not the case in mammalian oocytes. Because the spindle assembly portion of the SAC appears to function normally, two hypotheses have been proposed to explain the lack of response to univalents: (1) reduced stringency of the oocyte SAC to aberrant chromosome behavior, and (2) the ability of univalents to satisfy the SAC by forming bipolar attachments. The present study of Mlh1 mutant mice demonstrates that metaphase alignment is not a prerequisite for anaphase onset and provides strong evidence that MI spindle stabilization and anaphase onset require stable bipolar attachment of a critical mass--but not all--of chromosomes. We postulate that subtle differences in SAC-mediated control make the human oocyte inherently error prone and contribute to the age-related increase in aneuploidy.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
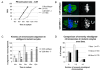

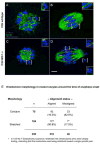
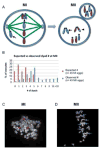
Comment in
-
Oogenesis: when most is good enough.Curr Biol. 2011 Apr 26;21(8):R288-90. doi: 10.1016/j.cub.2011.03.010. Curr Biol. 2011. PMID: 21514514
References
-
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. - PubMed
-
- Eaker S, Cobb J, Pyle A, Handel MA. Meiotic prophase abnormalities and metaphase cell death in MLH1-deficient mouse spermatocytes: insights into regulation of spermatogenic progress. Dev Biol. 2002;249:85–95. - PubMed
-
- Lipkin SM, Moens PB, Wang V, Lenzi M, Shanmugarajah D, Gilgeous A, Thomas J, Cheng J, Touchman JW, Green ED, et al. Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat Genet. 2002;31:385–390. - PubMed
-
- Burgoyne PS, Mahadevaiah SK, Turner JM. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet. 2009;10:207–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

