Cadmium is a catalytic inhibitor of DNA topoisomerase II
- PMID: 21497582
- PMCID: PMC3091975
- DOI: 10.1016/j.jinorgbio.2011.02.007
Cadmium is a catalytic inhibitor of DNA topoisomerase II
Abstract
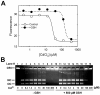
Cadmium (Cd(2+)) is a highly toxic and carcinogenic metal that is an environmental and occupational hazard. DNA topoisomerase II is an essential nuclear enzyme and its inhibition can result in the formation of genotoxic and recombinogenic DNA double strand breaks. In this study we showed that cadmium chloride strongly inhibited the DNA decatenation activity of human topoisomerase IIα in the low micromolar concentration range and that its inhibitory effects were reduced by glutathione. Because the activity of topoisomerase II is strongly inhibited by thiol-reactive compounds this result suggested that cadmium may be binding to critical topoisomerase II cysteine thiols. Cadmium, however, did not stabilize DNA-topoisomerase II covalent complexes, as measured by the lack of formation of DNA double strand breaks. Hence, it is not likely to be a topoisomerase II poison. Consistent with the idea that cadmium cytotoxicity may be modulated by glutathione levels, buthionine sulfoximine pretreatment to decrease glutathione levels resulted in a greatly increased cadmium-induced cytotoxicity in K562 cells. The results of this study suggest that cadmium may exert some of its cell growth inhibitory, and possibly its toxicity and carcinogenicity, by inhibiting topoisomerase IIα through reaction with critical cysteine thiols.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Bertin G, Averbeck D. Biochimie. 2006;88:1549–1559. - PubMed
-
- Jarup L, Akesson A. Toxicol. Appl. Pharmacol. 2009;238:201–208. - PubMed
-
- Giaginis C, Gatzidou E, Theocharis S. Toxicol. Appl. Pharmacol. 2006;213:282–290. - PubMed
-
- Satoh M, Koyama H, Kaji T, Kito H, Tohyama C. Tohoku J. Exp. Med. 2002;196:23–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

