Protein phosphatase 2A-SUR-6/B55 regulates centriole duplication in C. elegans by controlling the levels of centriole assembly factors
- PMID: 21497766
- PMCID: PMC3079880
- DOI: 10.1016/j.devcel.2011.03.007
Protein phosphatase 2A-SUR-6/B55 regulates centriole duplication in C. elegans by controlling the levels of centriole assembly factors
Abstract
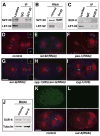
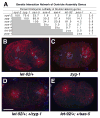
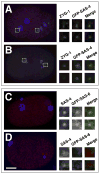
Centrioles play a crucial role in mitotic spindle assembly and duplicate precisely once per cell cycle. In worms, flies, and humans, centriole assembly is dependent upon a key regulatory kinase (ZYG-1/Sak/Plk4) and its downstream effectors SAS-5 and SAS-6. Here we report a role for protein phosphatase 2A (PP2A) in centriole duplication. We find that the PP2A catalytic subunit LET-92, the scaffolding subunit PAA-1, and the B55 regulatory subunit SUR-6 function together to positively regulate centriole assembly. In PP2A-SUR-6-depleted embryos, the levels of ZYG-1 and SAS-5 are reduced and the ZYG-1- and SAS-5-dependent recruitment of SAS-6 to the nascent centriole fails. We show that PP2A physically associates with SAS-5 in vivo and that inhibiting proteolysis can rescue SAS-5 levels and the centriole duplication defect of PP2A-depleted embryos. Together, our findings indicate that PP2A-SUR-6 promotes centriole assembly by protecting ZYG-1 and SAS-5 from degradation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

Comment in
-
PP2A targets SAS-5 in centriole assembly.Dev Cell. 2011 Apr 19;20(4):416-7. doi: 10.1016/j.devcel.2011.03.021. Dev Cell. 2011. PMID: 21497755 Free PMC article.
Similar articles
-
PP2A phosphatase acts upon SAS-5 to ensure centriole formation in C. elegans embryos.Dev Cell. 2011 Apr 19;20(4):550-62. doi: 10.1016/j.devcel.2011.02.005. Dev Cell. 2011. PMID: 21497765
-
The E2F-DP1 Transcription Factor Complex Regulates Centriole Duplication in Caenorhabditis elegans.G3 (Bethesda). 2016 Jan 15;6(3):709-20. doi: 10.1534/g3.115.025577. G3 (Bethesda). 2016. PMID: 26772748 Free PMC article.
-
The kinase ZYG-1 phosphorylates the cartwheel protein SAS-5 to drive centriole assembly in C. elegans.EMBO Rep. 2024 Jun;25(6):2698-2721. doi: 10.1038/s44319-024-00157-y. Epub 2024 May 14. EMBO Rep. 2024. PMID: 38744971 Free PMC article.
-
Ubiquitin signaling in the control of centriole duplication.FEBS J. 2022 Aug;289(16):4830-4849. doi: 10.1111/febs.16069. Epub 2021 Jun 29. FEBS J. 2022. PMID: 34115927 Review.
-
Dispatch. Centrosome biology: a SAS-sy centriole in the cell cycle.Curr Biol. 2003 Apr 29;13(9):R351-2. doi: 10.1016/s0960-9822(03)00273-2. Curr Biol. 2003. PMID: 12725749 Review.
Cited by
-
Revisiting Centrioles in Nematodes-Historic Findings and Current Topics.Cells. 2018 Aug 8;7(8):101. doi: 10.3390/cells7080101. Cells. 2018. PMID: 30096824 Free PMC article. Review.
-
PP2A targets SAS-5 in centriole assembly.Dev Cell. 2011 Apr 19;20(4):416-7. doi: 10.1016/j.devcel.2011.03.021. Dev Cell. 2011. PMID: 21497755 Free PMC article.
-
PIPKIγ targets to the centrosome and restrains centriole duplication.J Cell Sci. 2014 Mar 15;127(Pt 6):1293-305. doi: 10.1242/jcs.141465. Epub 2014 Jan 16. J Cell Sci. 2014. PMID: 24434581 Free PMC article.
-
PP2A regulatory subunit Bα controls endothelial contractility and vessel lumen integrity via regulation of HDAC7.EMBO J. 2013 Sep 11;32(18):2491-503. doi: 10.1038/emboj.2013.187. Epub 2013 Aug 16. EMBO J. 2013. PMID: 23955003 Free PMC article.
-
ATX-2, the C. elegans Ortholog of Human Ataxin-2, Regulates Centrosome Size and Microtubule Dynamics.PLoS Genet. 2016 Sep 30;12(9):e1006370. doi: 10.1371/journal.pgen.1006370. eCollection 2016 Sep. PLoS Genet. 2016. PMID: 27689799 Free PMC article.
References
-
- Castellanos E, Dominguez P, Gonzalez C. Centrosome dysfunction in Drosophila neural stem cells causes tumors that are not due to genome instability. Curr Biol. 2008;18:1209–1214. - PubMed
-
- Cunha-Ferreira I, Rodrigues-Martins A, Bento I, Riparbelli M, Zhang W, Laue E, Callaini G, Glover DM, Bettencourt-Dias M. The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr Biol. 2009;19:43–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

