Identification of unprecedented purine-containing compounds, the zingerines, from ginger rhizomes (Zingiber officinale Roscoe) using a phase-trafficking approach
- PMID: 21497863
- PMCID: PMC3142310
- DOI: 10.1016/j.phytochem.2011.03.007
Identification of unprecedented purine-containing compounds, the zingerines, from ginger rhizomes (Zingiber officinale Roscoe) using a phase-trafficking approach
Abstract
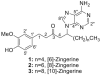
Three unprecedented purine-containing compounds, named [6]-, [8]-, and [10]-zingerines as they are 5-(6-amino-9H-purin-9-yl) analogs of [6]-, [8]-, and [10]-gingerols, respectively, were isolated from a methanolic extract of ginger rhizomes using a phase trafficking-based method that utilizes solid phase reagents allowing for fast and selective simultaneous separation of basic, acidic, and neutral components of natural products extracts.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


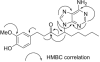

References
-
- Afzal M, Al-Hadidi D, Menon M, Pesek J, Dhami MSI. Ginger: an Ethomedical, Chemical And Pharmacological Review. Drug Metabol. Drug Interact. 2001;18:159–191. - PubMed
-
- Alexandratos SD. Ion-Exchange Resins: A Retrospective from Industrial and Engineering Chemistry Research. Ind. Eng. Chem. Res. 2009;48:388–398.
-
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008;46:409–420. - PubMed
-
- Appenzeller J, Mihci G, Martin MT, Gallard JF, Menou JL, Boury-Esnalllt N, Hooper J, Petek S, Chevalley S, Valentin A, Zaparucha A, Al-Mourabit A, Debitus C. Agelasines J, K, and L from the Solomon Islands marine sponge Agelas cf. mauritiana. J. Nat. Prod. 2008;71:1451–1454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

