Enhancing recovery from peripheral nerve injury using treadmill training
- PMID: 21498059
- PMCID: PMC3137663
- DOI: 10.1016/j.aanat.2011.02.013
Enhancing recovery from peripheral nerve injury using treadmill training
Abstract
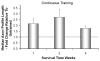
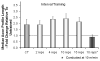
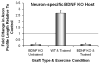
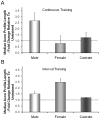
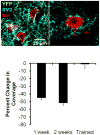
Full functional recovery after traumatic peripheral nerve injury is rare. We postulate three reasons for the poor functional outcome measures observed. Axon regeneration is slow and not all axons participate. Significant misdirection of regenerating axons to reinnervate inappropriate targets occurs. Seemingly permanent changes in neural circuitry in the central nervous system are found to accompany axotomy of peripheral axons. Exercise in the form of modest daily treadmill training impacts all three of these areas. Compared to untrained controls, regenerating axons elongate considerably farther in treadmill trained animals and do so via an autocrine/paracrine neurotrophin signaling pathway. This enhancement of axon regeneration takes place without an increase in the amount of misdirection of regenerating axons found without training. The enhancement also occurs in a sex-dependent manner. Slow continuous training is effective only in males, while more intense interval training is effective only in females. In treadmill trained, but not untrained mice the extent of coverage of axotomized motoneurons is maintained, thus preserving important elements of the spinal circuitry.
Copyright © 2011 Elsevier GmbH. All rights reserved.
Figures

References
-
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. - PubMed
-
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–48. - PubMed
-
- American Physiological Society. Resource Book for the Design of Animal Exercise Protocols. American Physiological Society; 2006.
-
- Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

