New strategies in Barrett's esophagus: integrating clonal evolutionary theory with clinical management
- PMID: 21498395
- PMCID: PMC3119197
- DOI: 10.1158/1078-0432.CCR-09-2358
New strategies in Barrett's esophagus: integrating clonal evolutionary theory with clinical management
Abstract
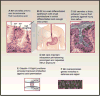
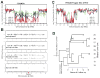
Barrett's esophagus is a condition in which the normal stratified squamous epithelium of the distal esophagus is replaced by intestinal metaplasia. For more than three decades, the prevailing clinical paradigm has been that Barrett's esophagus is a complication of symptomatic reflux disease that predisposes to esophageal adenocarcinoma. However, no clinical strategy for cancer prevention or early detection based on this paradigm has been proven to reduce esophageal adenocarcinoma mortality in a randomized clinical trial in part because only about 5% to 10% of individuals with Barrett's esophagus develop esophageal adenocarcinoma. Recent research indicates that Barrett's metaplasia is an adaptation for mucosal defense in response to chronic reflux in most individuals. The risk of progressing to esophageal adenocarcinoma is determined by development of genomic instability and dynamic clonal evolution in the distal esophagus modulated by host and environmental risk and protective factors, including inherited genotype. The challenge for investigators of Barrett's esophagus lies in integrating knowledge about genomic instability and clonal evolution into clinical management to increase the lifespan and quality of life of individuals with this condition.
©2011 AACR.
Figures
References
-
- Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97. - PubMed
-
- Barrett N. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br J Surg. 1950;38:175–82. - PubMed
-
- Naef AP, Savary M, Ozzello L. Columnar-lined lower esophagus: an acquired lesion with malignant predisposition. Report on 140 cases of Barrett’s esophagus with 12 adenocarcinomas. Journal of Thoracic and Cardiovascular Surgery. 1975;70:826–35. - PubMed
-
- Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 1998;93:1028–32. - PubMed
-
- Thomas T, Abrams KR, De Caestecker JS, Robinson RJ. Meta analysis: Cancer risk in Barrett’s oesophagus. Aliment Pharmacol Ther. 2007;26:1465–77. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

