Visual insight into how low pH alone can induce actin-severing ability in gelsolin under calcium-free conditions
- PMID: 21498516
- PMCID: PMC3121526
- DOI: 10.1074/jbc.M111.236943
Visual insight into how low pH alone can induce actin-severing ability in gelsolin under calcium-free conditions
Abstract
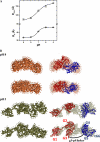
Gelsolin is a key actin cytoskeleton-modulating protein primarily regulated by calcium and phosphoinositides. In addition, low pH has also been suggested to activate gelsolin in the absence of Ca(2+) ions, although no structural insight on this pathway is available except for a reported decrement in its diffusion coefficient at low pH. We also observed ~1.6-fold decrease in the molecular mobility of recombinant gelsolin when buffer pH was lowered from 9 to 5. Analysis of the small angle x-ray scattering data collected over the same pH range indicated that the radius of gyration and maximum linear dimension of gelsolin molecules increased from 30.3 to 34.1 Å and from 100 to 125 Å, respectively. Models generated for each dataset indicated that similar to the Ca(2+)-induced process, low pH also promotes unwinding of this six-domain protein but only partially. It appeared that pH is able to induce extension of the G1 domain from the rest of the five domains, whereas the Ca(2+)-sensitive latch between G2 and G6 domains remains closed. Interestingly, increasing the free Ca(2+) level to merely ~40 nM, the partially open pH 5 shape "sprung open" to a shape seen earlier for this protein at pH 8 and 1 mm free Ca(2+). Also, pH alone could induce a shape where the g3-g4 linker of gelsolin was open when we truncated the C-tail latch from this protein. Our results provide insight into how under physiological conditions, a drop in pH can fully activate the F-actin-severing shape of gelsolin with micromolar levels of Ca(2+) available.
Figures
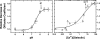




References
-
- Ashish, Paine M. S., Perryman P. B., Yang L., Yin H. L., Krueger J. K. (2007) J. Biol. Chem. 282, 25884–25892 - PubMed
-
- Yin H. L., Stossel T. P. (1979) Nature 281, 583–586 - PubMed
-
- Yin H. L. (1987) Bioessays 7, 176–179 - PubMed
-
- Sun H. Q., Yamamoto M., Mejillano M., Yin H. L. (1999) J. Biol. Chem. 274, 33179–33182 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

