Identification of the catalytic mechanism and estimation of kinetic parameters for fumarase
- PMID: 21498518
- PMCID: PMC3122171
- DOI: 10.1074/jbc.M110.214452
Identification of the catalytic mechanism and estimation of kinetic parameters for fumarase
Abstract
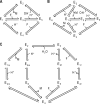
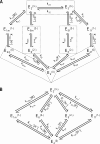
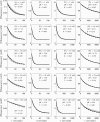
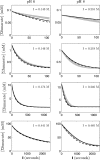
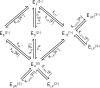
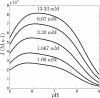
The enzyme fumarase catalyzes the reversible hydration of fumarate to malate. The reaction catalyzed by fumarase is critical for cellular energetics as a part of the tricarboxylic acid cycle, which produces reducing equivalents to drive oxidative ATP synthesis. A catalytic mechanism for the fumarase reaction that can account for the kinetic behavior of the enzyme observed in both isotope exchange studies and initial velocity studies has not yet been identified. In the present study, we develop an 11-state kinetic model of the enzyme based on the current consensus on its catalytic mechanism and design a series of experiments to estimate the model parameters and identify the major flux routes through the mechanism. The 11-state mechanism accounts for competitive binding of inhibitors and activation by different anions, including phosphate and fumarate. The model is identified from experimental time courses of the hydration of fumarate to malate obtained over a wide range of buffer and substrate concentrations. Further, the 11-state model is found to effectively reduce to a five-state model by lumping certain successive steps together to yield a mathematically less complex representation that is able to match the data. Analysis suggests the primary reaction route of the catalytic mechanism, with fumarate binding to the free unprotonated enzyme and a proton addition prior to malate release in the fumarate hydration reaction. In the reverse direction (malate dehydration), malate binds the protonated form of the enzyme, and a proton is generated before fumarate is released from the active site.
Figures
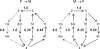
Similar articles
-
How fumarase recycles after the malate --> fumarate reaction. Insights into the reaction mechanism.Biochemistry. 1998 Dec 22;37(51):17651-8. doi: 10.1021/bi9821521. Biochemistry. 1998. PMID: 9922130
-
Kinetics of enzymes with iso-mechanisms: dead-end inhibition of fumarase and carbonic anhydrase II.Arch Biochem Biophys. 1994 Jul;312(1):227-33. doi: 10.1006/abbi.1994.1303. Arch Biochem Biophys. 1994. PMID: 8031132
-
Chicken fumarase. II. Kinetic studies.Biochimie. 1975;57(2):131-8. doi: 10.1016/s0300-9084(75)80162-3. Biochimie. 1975. PMID: 237577
-
Hyperpolarized [1,4-13C2]fumarate as an imaging agent of tumor cell death in vivo.2010 Jan 15 [updated 2010 Apr 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jan 15 [updated 2010 Apr 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641983 Free Books & Documents. Review.
-
Aspartase/fumarase superfamily: a common catalytic strategy involving general base-catalyzed formation of a highly stabilized aci-carboxylate intermediate.Biochemistry. 2012 May 29;51(21):4237-43. doi: 10.1021/bi300430j. Epub 2012 May 15. Biochemistry. 2012. PMID: 22551392 Review.
Cited by
-
Oxidative Stress and Antioxidant Defense in the Brain of Bat Species with Different Feeding Habits.Int J Mol Sci. 2023 Jul 29;24(15):12162. doi: 10.3390/ijms241512162. Int J Mol Sci. 2023. PMID: 37569536 Free PMC article.
-
Identification of the kinetic mechanism of succinyl-CoA synthetase.Biosci Rep. 2013 Jan 18;33(1):145-63. doi: 10.1042/BSR20120069. Biosci Rep. 2013. PMID: 23088689 Free PMC article.
-
Oxidative Stress and Antioxidant Defense in the Heart, Liver, and Kidney of Bat Species with Different Feeding Habits.Int J Mol Sci. 2023 Nov 15;24(22):16369. doi: 10.3390/ijms242216369. Int J Mol Sci. 2023. PMID: 38003558 Free PMC article.
-
Serum Metabolic Profiling Reveals the Antidepressive Effects of the Total Iridoids of Valeriana jatamansi Jones on Chronic Unpredictable Mild Stress Mice.Front Pharmacol. 2020 Mar 20;11:338. doi: 10.3389/fphar.2020.00338. eCollection 2020. Front Pharmacol. 2020. PMID: 32265710 Free PMC article.
-
Hypoxia in uterine fibroids: role in pathobiology and therapeutic opportunities.Oxygen (Basel). 2024 Jun;4(2):236-252. doi: 10.3390/oxygen4020013. Epub 2024 May 28. Oxygen (Basel). 2024. PMID: 38957794 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

