The initial phase of chromosome condensation requires Cdk1-mediated phosphorylation of the CAP-D3 subunit of condensin II
- PMID: 21498573
- PMCID: PMC3078710
- DOI: 10.1101/gad.2016411
The initial phase of chromosome condensation requires Cdk1-mediated phosphorylation of the CAP-D3 subunit of condensin II
Abstract
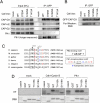
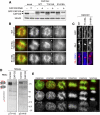
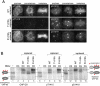
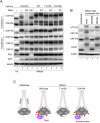
The cell cycle transition from interphase into mitosis is best characterized by the appearance of condensed chromosomes that become microscopically visible as thread-like structures in nuclei. Biochemically, launching the mitotic program requires the activation of the mitotic cyclin-dependent kinase Cdk1 (cyclin-dependent kinase 1), but whether and how Cdk1 triggers chromosome assembly at mitotic entry are not well understood. Here we report that mitotic chromosome assembly in prophase depends on Cdk1-mediated phosphorylation of the condensin II complex. We identified Thr 1415 of the CAP-D3 subunit as a Cdk1 phosphorylation site, which proved crucial as it was required for the Polo kinase Plk1 (Polo-like kinase 1) to localize to chromosome axes through binding to CAP-D3 and thereby hyperphosphorylate the condensin II complex. Live-cell imaging analysis of cells carrying nonphosphorylatable CAP-D3 mutants in place of endogenous protein suggested that phosphorylation of Thr 1415 is required for timely chromosome condensation during prophase, and that the Plk1-mediated phosphorylation of condensin II facilitates its ability to assemble chromosomes properly. These observations provide an explanation for how Cdk1 induces chromosome assembly in cells entering mitosis, and underscore the significance of the cooperative action of Plk1 with Cdk1.
Figures



References
-
- Archambault V, Glover DM 2009. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 10: 265–275 - PubMed
-
- Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB 2003. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 115: 83–95 - PubMed
-
- Gerlich D, Hirota T, Koch B, Peters JM, Ellenberg J 2006. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr Biol 16: 333–344 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
