Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes
- PMID: 21498611
- PMCID: PMC3175938
- DOI: 10.1167/iovs.11-7346
Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes
Abstract
Purpose: To evaluate the ability of mesenchymal stem cells (MSCs) engineered to produce and secrete brain-derived neurotrophic factor (BDNF) to protect retinal function and structure after intravitreal transplantation in a rat model of chronic ocular hypertension (COH).
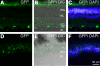
Methods: COH was induced by laser cauterization of trabecular meshwork and episcleral veins in rat eyes. COH eyes received an intravitreal transplant of MSCs engineered to express BDNF and green fluorescent protein (BDNF-MSCs) or just GFP (GFP-MSCs). Computerized pupillometry and electroretinography (ERG) were performed to assess optic nerve and retinal function. Quantification of optic nerve damage was performed by counting retinal ganglion cells (RGCs) and evaluating optic nerve cross-sections.
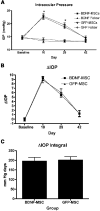
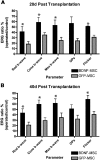
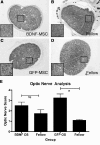
Results: After transplantation into COH eyes, BDNF-MSCs preserved significantly more retina and optic nerve function than GFP-MSC-treated eyes when pupil light reflex (PLR) and ERG function were evaluated. PLR analysis showed significantly better function (P = 0.03) in BDNF-MSC-treated eyes (operated/control ratio = 63.00% ± 11.39%) than GFP-MSC-treated eyes (operated/control ratio = 31.81% ± 9.63%) at 42 days after surgery. The BDNF-MSC-transplanted eyes also displayed a greater level of RGC preservation than eyes that received the GFP-MSCs only (RGC cell counts: BDNF-MSC-treated COH eyes, 112.2 ± 19.39 cells/section; GFP-MSC-treated COH eyes, 52.21 ± 11.54 cells/section; P = 0.01).
Conclusions: The authors have demonstrated that lentiviral-transduced BDNF-producing MSCs can survive in eyes with chronic hypertension and can provide retina and optic nerve functional and structural protection. Transplantation of BDNF-producing stem cells may be a viable treatment strategy for glaucoma.
Figures




Similar articles
-
Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma.Invest Ophthalmol Vis Sci. 2010 Apr;51(4):2051-9. doi: 10.1167/iovs.09-4509. Epub 2009 Nov 20. Invest Ophthalmol Vis Sci. 2010. PMID: 19933193 Free PMC article.
-
Functional evaluation of retina and optic nerve in the rat model of chronic ocular hypertension.Exp Eye Res. 2004 Jul;79(1):75-83. doi: 10.1016/j.exer.2004.02.011. Exp Eye Res. 2004. PMID: 15183102
-
Neuroprotection of Transplanting Human Umbilical Cord Mesenchymal Stem Cells in a Microbead Induced Ocular Hypertension Rat Model.Curr Eye Res. 2018 Jun;43(6):810-820. doi: 10.1080/02713683.2018.1440604. Epub 2018 Mar 5. Curr Eye Res. 2018. PMID: 29505314
-
Mesenchymal Stem Cell-Induced Neuroprotection in Pediatric Neurological Diseases: Recent Update of Underlying Mechanisms and Clinical Utility.Appl Biochem Biotechnol. 2024 Sep;196(9):5843-5858. doi: 10.1007/s12010-023-04752-y. Epub 2024 Jan 23. Appl Biochem Biotechnol. 2024. PMID: 38261236 Review.
-
Brain-Derived Neurotrophic Factor-Mediated Neuroprotection in Glaucoma: A Review of Current State of the Art.Front Pharmacol. 2022 May 20;13:875662. doi: 10.3389/fphar.2022.875662. eCollection 2022. Front Pharmacol. 2022. PMID: 35668928 Free PMC article. Review.
Cited by
-
Erythropoietin in Glaucoma: From Mechanism to Therapy.Int J Mol Sci. 2023 Feb 3;24(3):2985. doi: 10.3390/ijms24032985. Int J Mol Sci. 2023. PMID: 36769310 Free PMC article. Review.
-
Glaucoma, Stem Cells, and Gene Therapy: Where Are We Now?Int J Stem Cells. 2017 Nov 30;10(2):119-128. doi: 10.15283/ijsc17029. Int J Stem Cells. 2017. PMID: 28844129 Free PMC article. Review.
-
A novel glaucoma approach: Stem cell regeneration of the trabecular meshwork.Prog Retin Eye Res. 2022 Sep;90:101063. doi: 10.1016/j.preteyeres.2022.101063. Epub 2022 Apr 6. Prog Retin Eye Res. 2022. PMID: 35398015 Free PMC article. Review.
-
Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome.Brain. 2014 Feb;137(Pt 2):503-19. doi: 10.1093/brain/awt292. Epub 2013 Oct 30. Brain. 2014. PMID: 24176979 Free PMC article.
-
The Krüppel-Like Factor Gene Target Dusp14 Regulates Axon Growth and Regeneration.Invest Ophthalmol Vis Sci. 2018 Jun 1;59(7):2736-2747. doi: 10.1167/iovs.17-23319. Invest Ophthalmol Vis Sci. 2018. PMID: 29860460 Free PMC article.
References
-
- Nickells RT. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007;42:278–287 - PubMed
-
- Quigley HA, McKinnon S, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–3466 - PubMed
-
- Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105–114 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

