Antimicrobial effect of chitosan nanoparticles on streptococcus mutans biofilms
- PMID: 21498764
- PMCID: PMC3127608
- DOI: 10.1128/AEM.02941-10
Antimicrobial effect of chitosan nanoparticles on streptococcus mutans biofilms
Abstract
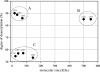
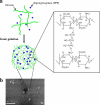
Nanoparticle complexes were prepared from chitosans of various molecular weights (MW) and degrees of deacetylation (DD). The antimicrobial effect was assessed by the Live/Dead BacLight technique in conjunction with confocal scanning laser microscopy (CSLM) and image analysis. Nanocomplexes prepared from chitosans with high MW showed a low antimicrobial effect (20 to 25% of cells damaged), whereas those prepared from low-MW chitosans showed high antimicrobial effect (>95% of cells damaged).
Figures

References
-
- Chávez de Paz L. E., Hamilton I. R., Svensäter G. 2008. Oral bacteria in biofilms exhibit slow reactivation from nutrient deprivation. Microbiology 154:1927–1938 - PubMed
-
- Hamilton I. R., Buckley N. D. 1991. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol. Immunol. 6:65–71 - PubMed
-
- Marsh P. D. 2004. Dental plaque as a microbial biofilm. Caries Res. 38:204–211 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

