AKAP signaling complexes in regulation of excitatory synaptic plasticity
- PMID: 21498812
- PMCID: PMC3126619
- DOI: 10.1177/1073858410384740
AKAP signaling complexes in regulation of excitatory synaptic plasticity
Abstract
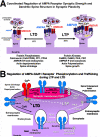
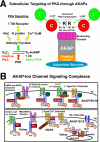
Plasticity at excitatory glutamatergic synapses in the central nervous system is believed to be critical for neuronal circuits to process and encode information, allowing animals to perform complex behaviors such as learning and memory. In addition, alterations in synaptic plasticity are associated with human diseases, including Alzheimer disease, epilepsy, chronic pain, drug addiction, and schizophrenia. Long-term potentiation (LTP) and depression (LTD) in the hippocampal region of the brain are two forms of synaptic plasticity that increase or decrease, respectively, the strength of synaptic transmission by postsynaptic AMPA-type glutamate receptors. Both LTP and LTD are induced by activation of NMDA-type glutamate receptors but differ in the level and duration of Ca(2+) influx through the NMDA receptor and the subsequent engagement of downstream signaling by protein kinases, including PKA, PKC, and CaMKII, and phosphatases, including PP1 and calcineurin-PP2B (CaN). This review addresses the important emerging roles of the A-kinase anchoring protein family of scaffold proteins in regulating localization of PKA and other kinases and phosphatases to postsynaptic multiprotein complexes that control NMDA and AMPA receptor function during LTP and LTD.
Figures






References
-
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272(52):32727–30. - PubMed
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276(5321):2042–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

