Drosophila melanogaster myosin-18 represents a highly divergent motor with actin tethering properties
- PMID: 21498886
- PMCID: PMC3122231
- DOI: 10.1074/jbc.M111.218669
Drosophila melanogaster myosin-18 represents a highly divergent motor with actin tethering properties
Abstract
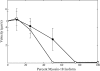
The gene encoding Drosophila myosin-18 is complex and can potentially yield six alternatively spliced mRNAs. One of the major features of this myosin is an N-terminal PDZ domain that is included in some of the predicted alternatively spliced products. To explore the biochemical properties of this protein, we engineered two minimal motor domain (MMD)-like constructs, one that contains the N-terminal PDZ (myosin-18 M-PDZ) domain and one that does not (myosin-18 M-ΔPDZ). These two constructs were expressed in the baculovirus/Sf9 system. The results suggest that Drosophila myosin-18 is highly divergent from most other myosins in the superfamily. Neither of the MMD constructs had an actin-activated MgATPase activity, nor did they even bind ATP. Both myosin-18 M-PDZ and M-ΔPDZ proteins bound to actin with K(d) values of 2.61 and 1.04 μM, respectively, but only about 50-75% of the protein bound to actin even at high actin concentrations. Unbound proteins from these actin binding assays reiterated the 60% saturation maximum, suggesting an equilibrium between actin-binding and non-actin-binding conformations of Drosophila myosin-18 in vitro. Neither the binding affinity nor the substoichiometric binding was significantly affected by ATP. Optical trapping of single molecules in three-bead assays showed short lived interactions of the myosin-18 motors with actin filaments. Combined, these data suggest that this highly divergent motor may function as an actin tethering protein.
Figures
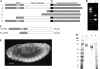
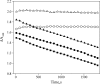

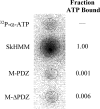

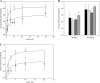
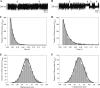
 ), the presence of 1 m
), the presence of 1 m

References
-
- Furusawa T., Ikawa S., Yanai N., Obinata M. (2000) Biochem. Biophys. Res. Commun. 270, 67–75 - PubMed
-
- Doyle D. A., Lee A., Lewis J., Kim E., Sheng M., MacKinnon R. (1996) Cell 85, 1067–1076 - PubMed
-
- Ajima R., Kajiya K., Inoue T., Tani M., Shiraishi-Yamaguchi Y., Maeda M., Segawa T., Furuichi T., Sutoh K., Yokota J. (2007) Biochem. Biophys. Res. Commun. 356, 851–856 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

