Characterization of Quin-C1 for its anti-inflammatory property in a mouse model of bleomycin-induced lung injury
- PMID: 21499285
- PMCID: PMC4002518
- DOI: 10.1038/aps.2011.4
Characterization of Quin-C1 for its anti-inflammatory property in a mouse model of bleomycin-induced lung injury
Abstract
Aim: To study the in vivo effects of Quin-C1, a highly specific agonist for formyl peptide receptor 2 (FPR2/ALX), in a mouse model of bleomycin (BLM)-induced lung injury.
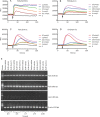
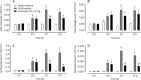
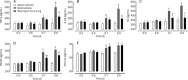
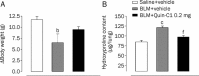
Methods: Male ICR mice were injected intratracheally with BLM (d 0), and intraperitoneally with Quin-C1 (0.2 mg/d) or vehicle between d 1 and d 28, during which pulmonary inflammation was monitored. A similar regimen was carried out between d 5 and d 28 to differentiate anti-inflammatory from anti-fibrotic effects. During the treatment, leukocyte numbers in bronchoalveolar lavage fluid (BALF) were counted, and FPR2/ALX transcripts, tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), the mouse keratinocyte-derived chemokine (KC), transforming growth factor β1 (TGF-β1) and C-X-C motif chemokine 10 (CXCL10) expression levels in the lung tissue were also measured. Both hydroxyproline content and histological changes were examined on d 28 to assess the severity of lung fibrosis.
Results: BLM caused a significant increase in expression levels of all the selected cytokines and chemokines, as well as a thickening of the alveolar wall. Treatment with Quin-C1 significantly reduced the neutrophil and lymphocyte counts in BALF, diminished expression of TNF-α, IL-1β, KC, and TGF-β1, and decreased collagen deposition in lung tissue. The treatment also lowered the content of lung hydroxyproline. Quin-C1 did not ameliorate lung fibrosis when the treatment was started 5 d after the BLM challenge, suggesting that the protection may be attributed to its anti-inflammatory effects. Exposure to BLM or BLM plus Quin-C1 did not change the level of FPR2/ALX transcripts (mFpr1, mFpr2, and Lxa4r) in the lung tissue.
Conclusion: The results demonstrate an anti-inflammatory role for Quin-C1 in bleomycin-induced lung injury, which may be further explored for therapeutic applications.
Figures

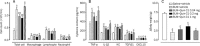
Similar articles
-
Oral FPR2/ALX modulators tune myeloid cell activity to ameliorate mucosal inflammation in inflammatory bowel disease.Acta Pharmacol Sin. 2025 Jul;46(7):1958-1973. doi: 10.1038/s41401-025-01525-7. Epub 2025 Mar 11. Acta Pharmacol Sin. 2025. PMID: 40069490
-
[Effects of andrographolide on the concentration of cytokines in BALF and the expressions of type I and III collagen mRNA in lung tissue in bleomycin-induced rat pulmonary fibrosis].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011 Jul;27(7):725-9. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011. PMID: 21722520 Chinese.
-
Terrestrosin D from Tribulus terrestris attenuates bleomycin-induced inflammation and suppresses fibrotic changes in the lungs of mice.Pharm Biol. 2019 Dec;57(1):694-700. doi: 10.1080/13880209.2019.1672754. Pharm Biol. 2019. PMID: 31608748 Free PMC article.
-
Essential oil from Inula japonica Thunb. And its phenolic constituents ameliorate pulmonary injury and fibrosis in bleomycin-treated mice.J Ethnopharmacol. 2024 Jan 30;319(Pt 1):117169. doi: 10.1016/j.jep.2023.117169. Epub 2023 Sep 11. J Ethnopharmacol. 2024. PMID: 37704119
-
Asiatic acid ameliorates pulmonary fibrosis induced by bleomycin (BLM) via suppressing pro-fibrotic and inflammatory signaling pathways.Biomed Pharmacother. 2017 May;89:1297-1309. doi: 10.1016/j.biopha.2017.03.005. Epub 2017 Mar 17. Biomed Pharmacother. 2017. PMID: 28320097
Cited by
-
Targeting of Formyl Peptide Receptor 2 for in vivo imaging of acute vascular inflammation.Theranostics. 2020 May 17;10(15):6599-6614. doi: 10.7150/thno.44226. eCollection 2020. Theranostics. 2020. PMID: 32550892 Free PMC article.
-
Developmental and homeostatic signaling transmitted by the G-protein coupled receptor FPR2.Int Immunopharmacol. 2023 May;118:110052. doi: 10.1016/j.intimp.2023.110052. Epub 2023 Mar 30. Int Immunopharmacol. 2023. PMID: 37003185 Free PMC article. Review.
-
Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists.Int J Mol Sci. 2013 Apr 2;14(4):7193-230. doi: 10.3390/ijms14047193. Int J Mol Sci. 2013. PMID: 23549262 Free PMC article. Review.
-
Chemotactic Ligands that Activate G-Protein-Coupled Formylpeptide Receptors.Int J Mol Sci. 2019 Jul 12;20(14):3426. doi: 10.3390/ijms20143426. Int J Mol Sci. 2019. PMID: 31336833 Free PMC article. Review.
-
Novel ureidopropanamide based N-formyl peptide receptor 2 (FPR2) agonists with potential application for central nervous system disorders characterized by neuroinflammation.Eur J Med Chem. 2017 Dec 1;141:703-720. doi: 10.1016/j.ejmech.2017.09.023. Epub 2017 Sep 18. Eur J Med Chem. 2017. PMID: 29102463 Free PMC article.
References
-
- Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–19. - PubMed
-
- Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35:2486–95. - PubMed
-
- Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

