The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers
- PMID: 21499315
- PMCID: PMC3287066
- DOI: 10.1038/nmat2992
The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers
Erratum in
- Nat Mater. 2011 Jun;10(6):476
Abstract
Encapsulation of drugs within nanocarriers that selectively target malignant cells promises to mitigate side effects of conventional chemotherapy and to enable delivery of the unique drug combinations needed for personalized medicine. To realize this potential, however, targeted nanocarriers must simultaneously overcome multiple challenges, including specificity, stability and a high capacity for disparate cargos. Here we report porous nanoparticle-supported lipid bilayers (protocells) that synergistically combine properties of liposomes and nanoporous particles. Protocells modified with a targeting peptide that binds to human hepatocellular carcinoma exhibit a 10,000-fold greater affinity for human hepatocellular carcinoma than for hepatocytes, endothelial cells or immune cells. Furthermore, protocells can be loaded with combinations of therapeutic (drugs, small interfering RNA and toxins) and diagnostic (quantum dots) agents and modified to promote endosomal escape and nuclear accumulation of selected cargos. The enormous capacity of the high-surface-area nanoporous core combined with the enhanced targeting efficacy enabled by the fluid supported lipid bilayer enable a single protocell loaded with a drug cocktail to kill a drug-resistant human hepatocellular carcinoma cell, representing a 10(6)-fold improvement over comparable liposomes.
Figures



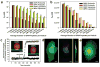

Comment in
-
Drug delivery: One nanoparticle, one kill.Nat Mater. 2011 May;10(5):342-3. doi: 10.1038/nmat3014. Nat Mater. 2011. PMID: 21499312 Free PMC article.
Similar articles
-
Delivery of ricin toxin a-chain by peptide-targeted mesoporous silica nanoparticle-supported lipid bilayers.Adv Healthc Mater. 2012 May;1(3):348-53. doi: 10.1002/adhm.201200022. Epub 2012 Apr 2. Adv Healthc Mater. 2012. PMID: 23184753 Free PMC article.
-
Protocells: Modular Mesoporous Silica Nanoparticle-Supported Lipid Bilayers for Drug Delivery.Small. 2016 Apr 27;12(16):2173-85. doi: 10.1002/smll.201502119. Epub 2016 Jan 18. Small. 2016. PMID: 26780591 Free PMC article. Review.
-
Delivery of small interfering RNA by peptide-targeted mesoporous silica nanoparticle-supported lipid bilayers.ACS Nano. 2012 Mar 27;6(3):2174-88. doi: 10.1021/nn204102q. Epub 2012 Feb 14. ACS Nano. 2012. PMID: 22309035 Free PMC article.
-
Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility.Acc Chem Res. 2013 Mar 19;46(3):792-801. doi: 10.1021/ar3000986. Epub 2013 Feb 6. Acc Chem Res. 2013. PMID: 23387478 Free PMC article.
-
Nanoparticles for targeted delivery of therapeutics and small interfering RNAs in hepatocellular carcinoma.World J Gastroenterol. 2015 Nov 14;21(42):12022-41. doi: 10.3748/wjg.v21.i42.12022. World J Gastroenterol. 2015. PMID: 26576089 Free PMC article. Review.
Cited by
-
Photodynamic-Chemodynamic Cascade Reactions for Efficient Drug Delivery and Enhanced Combination Therapy.Adv Sci (Weinh). 2021 Apr 8;8(10):2002927. doi: 10.1002/advs.202002927. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026433 Free PMC article.
-
Integration of Chinese medicine with Western medicine could lead to future medicine: molecular module medicine.Chin J Integr Med. 2016 Apr;22(4):243-50. doi: 10.1007/s11655-016-2495-0. Epub 2016 Apr 9. Chin J Integr Med. 2016. PMID: 27059483 Review.
-
Azeotropic Distillation-Induced Self-Assembly of Mesostructured Spherical Nanoparticles as Drug Cargos for Controlled Release of Curcumin.Pharmaceuticals (Basel). 2022 Feb 23;15(3):275. doi: 10.3390/ph15030275. Pharmaceuticals (Basel). 2022. PMID: 35337073 Free PMC article.
-
Multifunctional Lipid Bilayer Nanocarriers for Cancer Immunotherapy in Heterogeneous Tumor Microenvironments, Combining Immunogenic Cell Death Stimuli with Immune Modulatory Drugs.ACS Nano. 2022 Apr 26;16(4):5184-5232. doi: 10.1021/acsnano.2c01252. Epub 2022 Mar 29. ACS Nano. 2022. PMID: 35348320 Free PMC article. Review.
-
Polysilsesquioxane nanoparticles for targeted platin-based cancer chemotherapy by triggered release.Angew Chem Int Ed Engl. 2011 Oct 24;50(44):10330-4. doi: 10.1002/anie.201104510. Epub 2011 Sep 14. Angew Chem Int Ed Engl. 2011. PMID: 21915976 Free PMC article. No abstract available.
References
-
- Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2:751–760. - PubMed
-
- Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotech. 2006;24:1211–1217. - PubMed
-
- Nel AE, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. - PubMed
-
- Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nature Reviews Cancer. 2005;5:161–171. - PubMed
-
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release. 2000;65:271–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

